

| % CHANGE | |||||
|---|---|---|---|---|---|
| Millions (Except Per Common Share Data) | 2015 | 2014 | 2013 | '15/'14 | '14/'13 |
| Revenues(a) | $48,851 | $49,605 | $51,584 | (2) | (4) |
| Research and development expenses(a) | 7,690 | 8,393 | 6,678 | (8) | 26 |
| Restructuring charges and certain acquisition-related costs(a) | 1,152 | 250 | 1,182 | * | (79) |
| Income from continuing operations(a) | 6,975 | 9,119 | 11,410 | (24) | (20) |
| Discontinued operations — net of tax(b) | 11 | 48 | 10,662 | (77) | (100) |
| Net income attributable to Pfizer Inc.(a) | 6,960 | 9,135 | 22,003 | (24) | (58) |
| Diluted earnings per common share attributable to Pfizer Inc. shareholders | 1.11 | 1.42 | 3.19 | (22) | (55) |
| Weighted-average shares — diluted | 6,257 | 6,424 | 6,895 | (3) | (7) |
| Number of common shares outstanding | 6,175 | 6,291 | 6,399 | (2) | (2) |
| Total assets(d) | 167,460 | 167,566 | 170,415 | — | (2) |
| Total long-term obligations(a), (c), (d) | 73,064 | 74,357 | 73,801 | (2) | 1 |
| Total Pfizer Inc. shareholders' equity | 64,720 | 71,301 | 76,307 | (9) | (7) |
| Shareholders' equity per common share | 10.48 | 11.33 | 11.93 | (8) | (5) |
| Net cash provided by operating activities | 14,512 | 16,883 | 17,684 | (14) | (5) |
| Property, plant and equipment additions | 1,397 | 1,199 | 1,206 | 17 | (1) |
| Purchases of common stock | 6,160 | 5,000 | 16,290 | 23 | (69) |
| Cash dividends paid | 6,940 | 6,609 | 6,580 | 5 | — |
* Calculation not meaningful
(a) In accordance with our domestic and international reporting periods, amounts for 2015 reflect four months of legacy Hospira U.S. operations and three months of legacy Hospira international operations. Amounts for 2013 reflect the June 24, 2013, disposition of Zoetis, Inc. and its presentation as a discontinued operation.
(b) Includes the Animal Health (Zoetis, Inc.) business through June 24, 2013, the date of disposal.
(c) Defined as Long-term debt, Pension benefit obligations, net, Postretirement benefit obligations, net, Noncurrent deferred tax liabilities, Other taxes payable and Other noncurrent liabilities. Our short-term borrowings are rated P-1 by Moody's Investors Service (Moody's) and A-1+ by Standard & Poor's (S&P). Our long-term debt is rated A1 by Moody's (Outlook: Stable) and AA by S&P (Outlook: Negative Watch). Moody's and S&P are major corporate debt-rating organizations. A security rating is not a recommendation to buy, sell or hold securities and the rating is subject to revision or withdrawal at any time by the rating organization. Each rating should be evaluated independently of any other rating.
(d) All amounts reflect the retrospective adoption of a new accounting standard as of December 31, 2015, that requires all deferred tax assets and liabilities to be classified as noncurrent in the balance sheet.
Detailed information on our financial and operational performance can be found in the 2015 Financial Report, which is filed as Exhibit 13 to our 2015 Annual Report on Form 10-K.
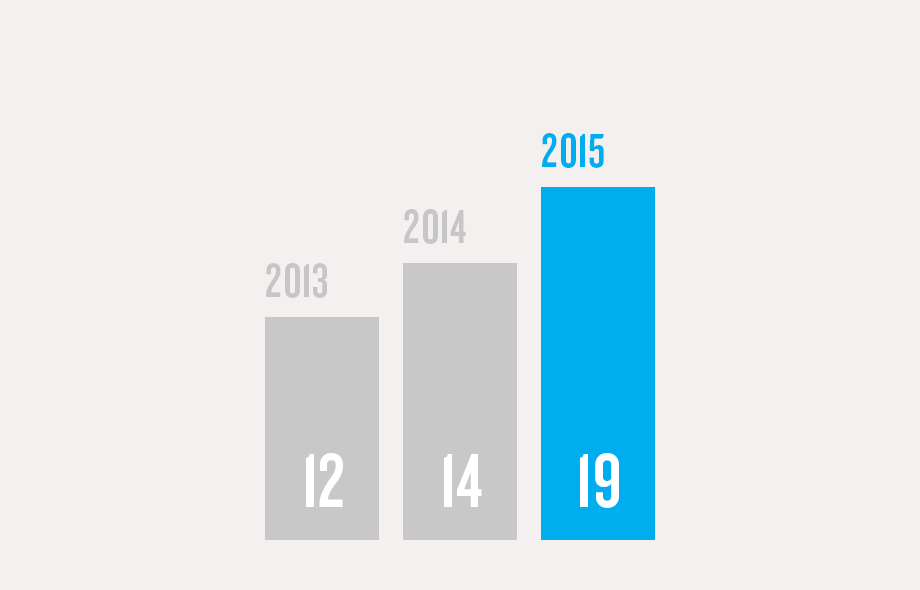
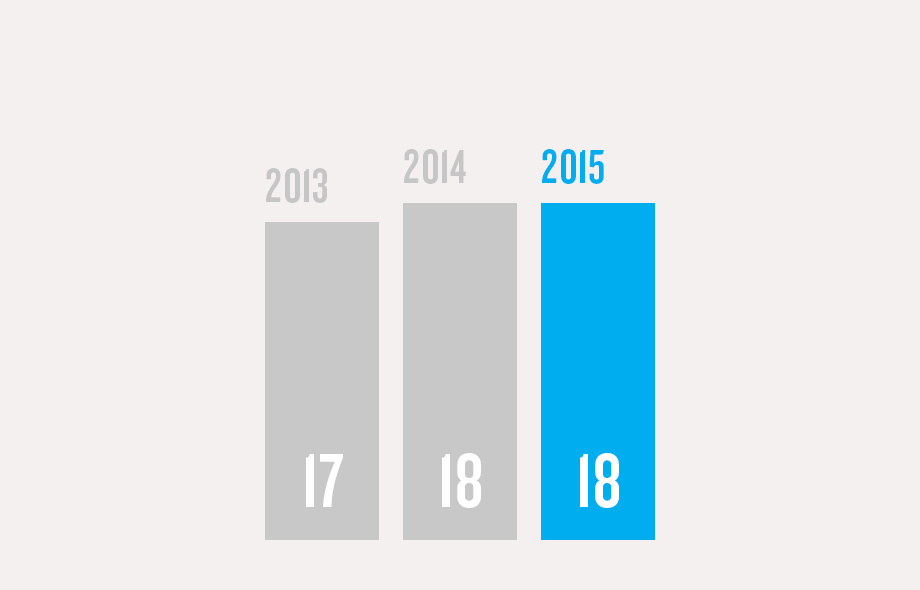
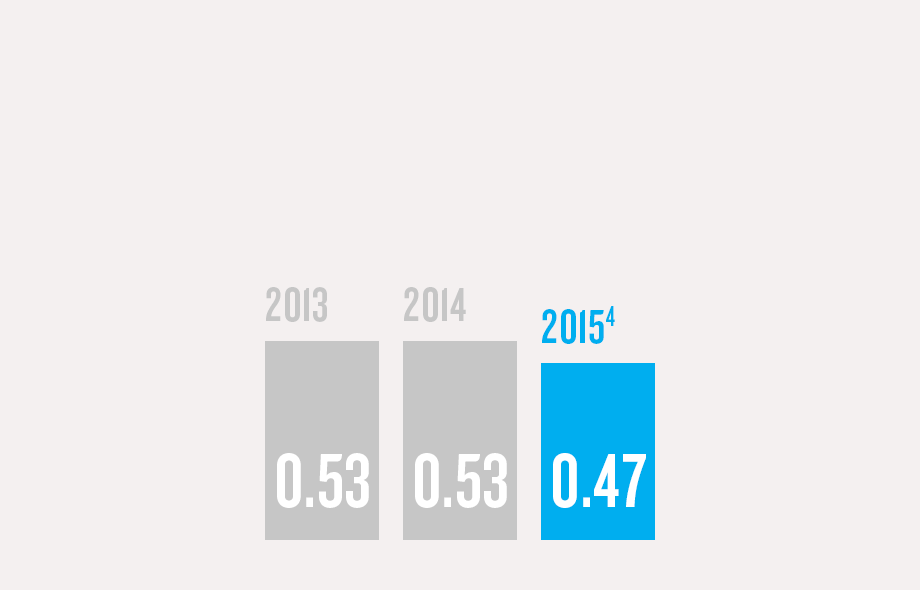
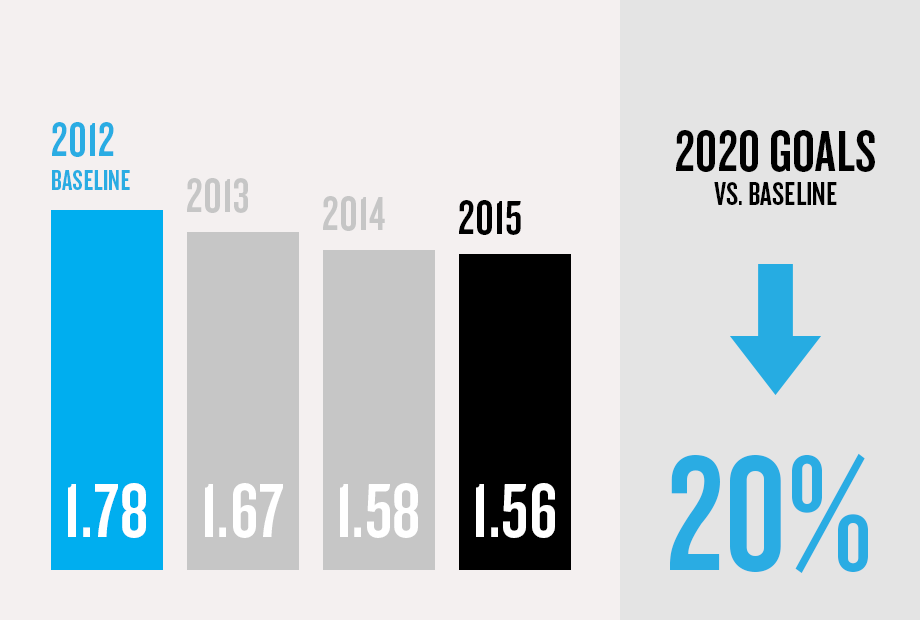
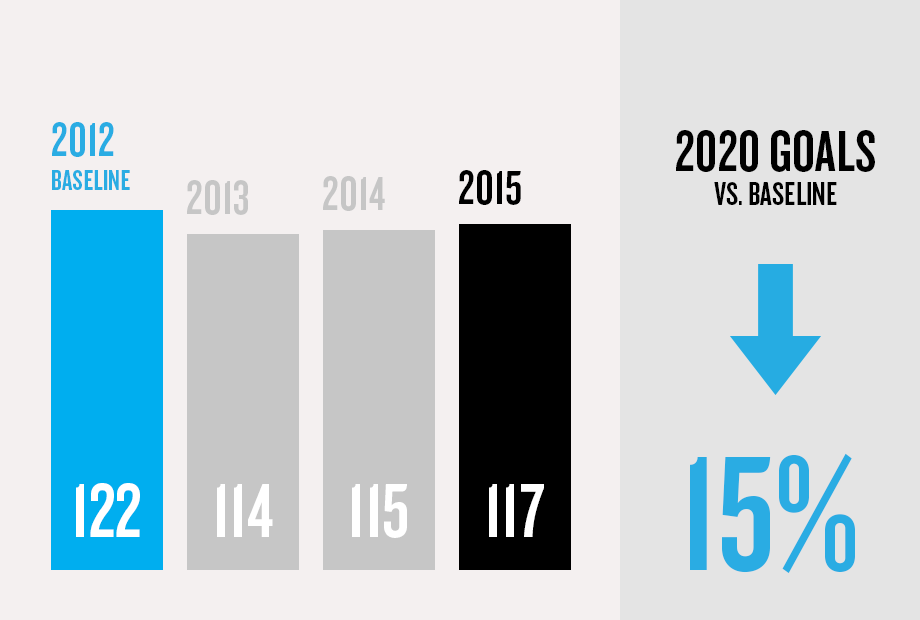
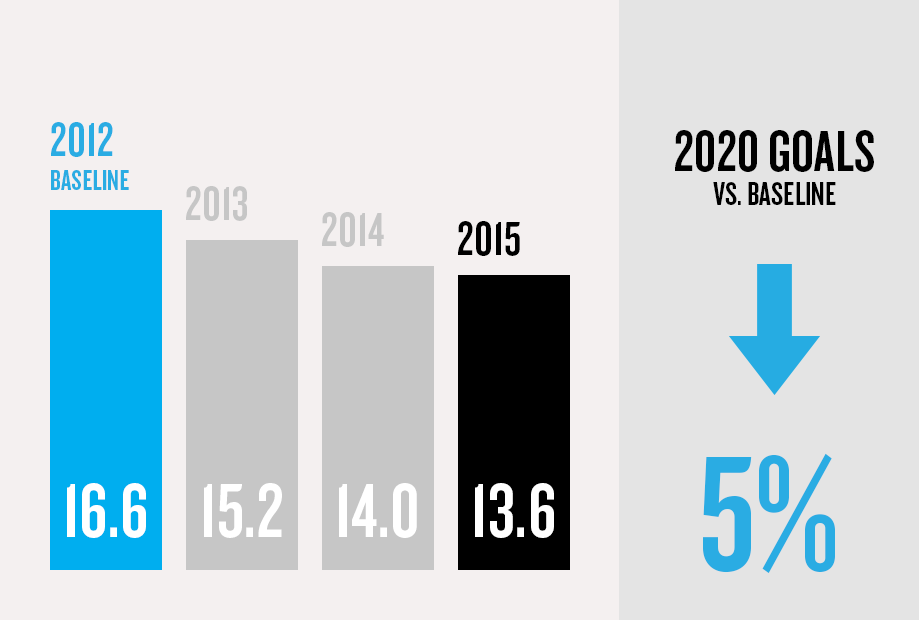
(1) Program/commercial transaction defined as a Pfizer investment or dedicated contract of over $250,000 with a national government or procurement agency, multilateral organization, non-governmental organization, private institution or aid agency. Represents multi-country initiatives only and does not include numerous local initiatives to address access.
(2) As defined by the World Health Organization. Burdens of illness not addressed include road traffic accidents, prematurity and low birth weight, and self-inflicted injuries.
(3) The number of patient access programs with pricing tailored to different patient segments (for at least one product), allowing access for more patients.
(4) Hospira injury data is not included. Combined company data will be provided in the 2016 Annual Review.
(5) Applies to facilities within Pfizer's operational control as compared with a 2012 baseline. Hospira environmental sustainability data is not included. Combined company data will be provided in the 2016 Annual Review.
Data are baseline adjusted, reported absolute, using reporting boundaries per the
WRI GHG Protocol. The 2012–2014
GHG data was independently verified to the limited assurance
level. The verification of the 2015 GHG data will be accomplished in
2016. Expanded environmental reporting will be posted on www.pfizer.com later this year.
(1) Please refer to Pfizer's 2015 Annual Report on Form 10-K, including the sections captioned Risk Factors
and Forward-Looking Information and Factors That May Affect Future Results,
for a description of the substantial risks and uncertainties related to the forward-looking statements, including our 2016 Financial Guidance, included in this Annual Review.
(2) Our 2015 financial guidance was at exchange rates that reflected a blend of the actual exchange rates in effect through September 27, 2015, and the mid-October 2015 exchange rates for the remainder of the year and excluded the potential impact of a devaluation of the Venezuelan bolivar. Our 2015 guidance did not assume the completion of any business development transactions not completed as of September 27, 2015, including any one-time upfront payments associated with such transactions, and excluded the potential effects of the resolution of litigation-related matters not substantially resolved as of September 27, 2015. Guidance for Reported Revenues reflected the anticipated negative impact of $3.3 billion due to products that have recently lost patent protection. Reported and Adjusted Diluted EPS guidance assumed diluted weighted-average shares outstanding of ~6.25 billion shares. Additional disclosures and assumptions regarding our 2015 financial guidance can be found in Pfizer's Current Report on Form 8-K dated October 28, 2015.
(3) The 2016 financial guidance was issued in February 2016 and reflects the following:
Does not assume the completion of any business development transactions not completed as of December 31, 2015, including any one-time upfront payments associated with such transactions. 2016 financial guidance excludes any impact from the pending combination with Allergan. The transaction is expected to close during the second half of 2016.
Excludes the potential effects of the resolution of litigation-related matters not substantially resolved as of February 12, 2016.
Exchange rates assumed are as of mid-January 2016.
Guidance for 2016 reported revenues reflects the anticipated negative impact of $2.3 billion due to recent and expected generic competition for certain products that have recently lost or are anticipated to soon lose patent protection.
Guidance for 2016 reported revenues also reflects the anticipated negative impact of $2.3 billion as a result of unfavorable changes in foreign exchange rates relative to the U.S. dollar compared to foreign exchange rates from 2015, including $0.8 billion due to the estimated significant negative currency impact related to Venezuela. The anticipated negative impact on reported and adjusted diluted EPS resulting from unfavorable changes in foreign exchange rates compared to foreign exchange rates from 2015 is approximately $0.16, including $0.07 due to the estimated significant negative currency impact related to Venezuela.
Guidance for reported and adjusted diluted EPS assumes diluted weighted-average shares outstanding of ~6.2 billion shares.
(4) Adjusted Income
and its components and Adjusted Diluted Earnings Per Share (EPS)
are defined as reported U.S. generally accepted accounting principles (U.S. GAAP) net income(5) and its components and reported diluted EPS(5) excluding purchase accounting adjustments, acquisition-related costs, discontinued operations and certain significant items. Adjusted Revenues, Adjusted Cost of Sales, Adjusted Selling, Informational and Administrative (SI&A), Adjusted Research and Development (R&D) expenses and Adjusted Other (Income)/Deductions are income statement line items prepared on the same basis as, and therefore components of, the overall Adjusted income measure. As described under Adjusted Income
in the Management's Discussion and Analysis of Financial Condition and Results of Operations section of our Annual Report on Form 10-K for the year ended December 31, 2015, management uses Adjusted income, among other factors, to set performance goals and to measure the performance of the overall company. We believe that investors' understanding of our performance is enhanced by disclosing this measure. Reconciliations of certain U.S. GAAP Reported to Non-GAAP Adjusted information for 2015, as well as reconciliations of full-year 2016 guidance for Adjusted income and Adjusted diluted EPS to full-year 2016 guidance for Reported net income(5) and Reported diluted EPS,(5) are provided in the Management's Discussion and Analysis of Financial Condition and Results of Operations section of our Annual Report on Form 10-K for the year ended December 31, 2015. The Adjusted income and its components and Adjusted diluted EPS measures are not, and should not be viewed as, substitutes for U.S. GAAP net income and its components and diluted EPS. Despite the importance of these measures to management in goal setting and performance measurement, Adjusted income and its components and Adjusted diluted EPS are Non-GAAP financial measures that have no standardized meaning prescribed by U.S. GAAP and, therefore, have limits in their usefulness to investors. Because of the non-standardized definitions, Adjusted income and its components and Adjusted diluted EPS (unlike U.S. GAAP net income and its components and diluted EPS) may not be comparable to the calculation of similar measures of other companies. Adjusted income and its components and Adjusted diluted EPS are presented solely to permit investors to more fully understand how management assesses performance.
(5) Reported Net Income
in accordance with U.S. GAAP is defined as net income attributable to Pfizer Inc. in accordance with U.S. GAAP and Reported Diluted EPS
is defined as reported diluted EPS attributable to Pfizer Inc. common shareholders in accordance with U.S. GAAP.