
1849
Cousins Charles Pfizer and Charles Erhart founded Charles Pfizer & Company in a red brick building in Brooklyn, NY.

Associate Research Fellow and Group Leader, Chemical Development Active Pharmaceutical Ingredients
Senior Director, Oncology Medicinal Chemistry Synthesis and Analytical Group
Associate Research Fellow and Group Leader, Chemical Development Active Pharmaceutical Ingredients
Senior Director, Oncology Medicinal Chemistry Synthesis and Analytical Group
Associate Research Fellow and Group Leader, Chemical Development Active Pharmaceutical Ingredients
Senior Director, Oncology Medicinal Chemistry Synthesis and Analytical Group
Associate Research Fellow and Group Leader, Chemical Development Active Pharmaceutical Ingredients
Senior Director, Oncology Medicinal Chemistry Synthesis and Analytical Group

Cousins Charles Pfizer and Charles Erhart founded Charles Pfizer & Company in a red brick building in Brooklyn, NY.

Pfizer buys and renovates a post-Revolutionary-era building at 81 Maiden Lane in Manhattan and moves its headquarters there.
The expansion propelled by the Civil War continues and Pfizer's revenues double.
The company now has a substantially increased product line and 150 new employees. To accommodate this growth, it buys and renovates a post-Revolutionary-era building at 81 Maiden Lane in Manhattan and moves its headquarters there. The site carries the Pfizer name for nearly a century.
Collapse
Spurred by America's westward expansion and its own growing number of clients west of the Mississippi, Pfizer opens offices and a warehouse in Chicago, Illinois, its first location outside of New York.

Pfizer files an official certificate of incorporation in the state of New Jersey, with authorized capital of $2 million divided into 20,000 shares of $100 each.
Pfizer would remain a privately held company until June 22, 1942, when 240,000 shares of new common stock were offered to the public.
Collapse
Emile Pfizer was the last member of the Pfizer/Erhart family to be actively involved with the company. He was president from 1906 to 1941.
Emile Pfizer, Charles Pfizer's youngest son, is appointed President at a special board meeting. He serves as President from 1906 to 1941 and briefly as Chairman in 1941. He is the last member of the Pfizer/Erhart family to be actively involved with the company.
Collapse
At the age of 82, Charles Pfizer dies while vacationing at his Newport, Rhode Island estate. A tribute to Pfizer in The New York Tribune notes that "by bringing to his task a thorough German technical education, great industry, and determination, he successfully met all difficulties and each year expanded his business." Company sales exceed $3 million.

John Anderson, starting at Pfizer in 1873 as an office boy, opened the company's first branch in Chicago within ten years. Later he served as Chairman of the Board, 1914-1929.
The Board of Directors creates the position of Chairman and elects John Anderson to that post. Anderson, who had joined Pfizer in 1873 as a 16-year-old office boy, would remain Chairman until 1929.
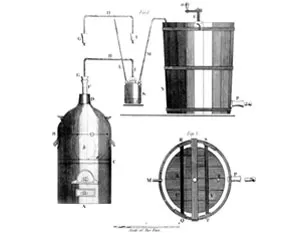
Pfizer chemist James Currie and his assistant, Jasper Kane, successfully pioneer the mass production of citric acid from sugar through mold fermentation—an achievement that eventually frees Pfizer from dependency on European citrus growers.
Spurred by this invention, Kane goes on to develop a new deep-tank fermentation method using molasses rather than refined sugar as raw material—the process that will ultimately unlock the secret for large-scale production of penicillin.
Collapse
Charles Pfizer & Co. turns 75 years old. A celebration at the Brooklyn plant, which has 306 employees, marks the milestone.

William H. Erhart, son of Charles Erhart, one of the two original founders. He became vice president of the company in 1900 and served as Chairman of the Board from 1929 to 1940.
On January 10, 1929, John Anderson announces he is stepping down as chairman of the board. William Erhart is named the new chairman, Emile Pfizer continues to serve as president, and John Anderson's son, George, becomes senior vice president.
Collapse
Doctor Richard Pasternack develops a fermentation-free method for producing ascorbic acid, vitamin C. After building a new plant and initiating a 24-hour-a-day, seven-day-a-week production schedule, Pfizer becomes the world's leading producer of vitamin C.
Encouraged by this success, Pfizer pushes ahead in 1938 with production of vitamin B-2, or riboflavin, and eventually develops a vitamin mix that includes riboflavin, thiamin, niacin, and iron. From vitamin B-12, the company moves on to vitamin A, and by the late 1940s, Pfizer will become the established leader in the manufacture of vitamins.
Collapse
Pfizer succeeds so well in the production of citric acid by fermenting sugar that a pound of citric acid, which had cost $1.25 in 1919, tumbles to 20¢, and Pfizer is widely recognized as a leader in fermentation technology.
Pfizer responds to an appeal from the United States Government to expedite the manufacture of penicillin to treat Allied soldiers fighting in World War II. Of the companies pursuing mass production of penicillin, Pfizer alone uses fermentation technology.
In a risky maneuver, Pfizer's senior management invests millions of dollars, putting their own assets as Pfizer stockholders at stake, to buy the equipment and facilities needed for this novel process of deep-tank fermentation. Pfizer purchases a nearby vacant ice plant, and employees work around the clock to convert it and perfect the complex production process. In just four months, Pfizer is producing five times more penicillin than originally anticipated. Penicillin is a turning point in human history—the first real defense against bacterial infection.
Collapse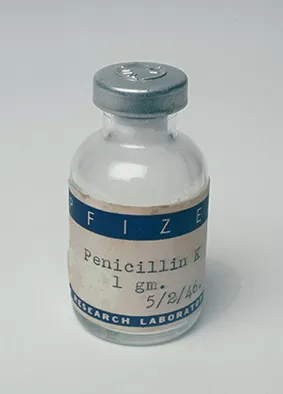
Pfizer is successful in its efforts to mass-produce penicillin and becomes the world's largest producer of the "miracle drug."
Using deep-tank fermentation, Pfizer is successful in its efforts to mass-produce penicillin and becomes the world's largest producer of the "miracle drug."
Most of the penicillin that goes ashore with Allied forces on D-Day is made by Pfizer. The company's contribution to the war effort is heralded nationwide and earns Pfizer the coveted Army-Navy "E" Award on April 17, 1943.
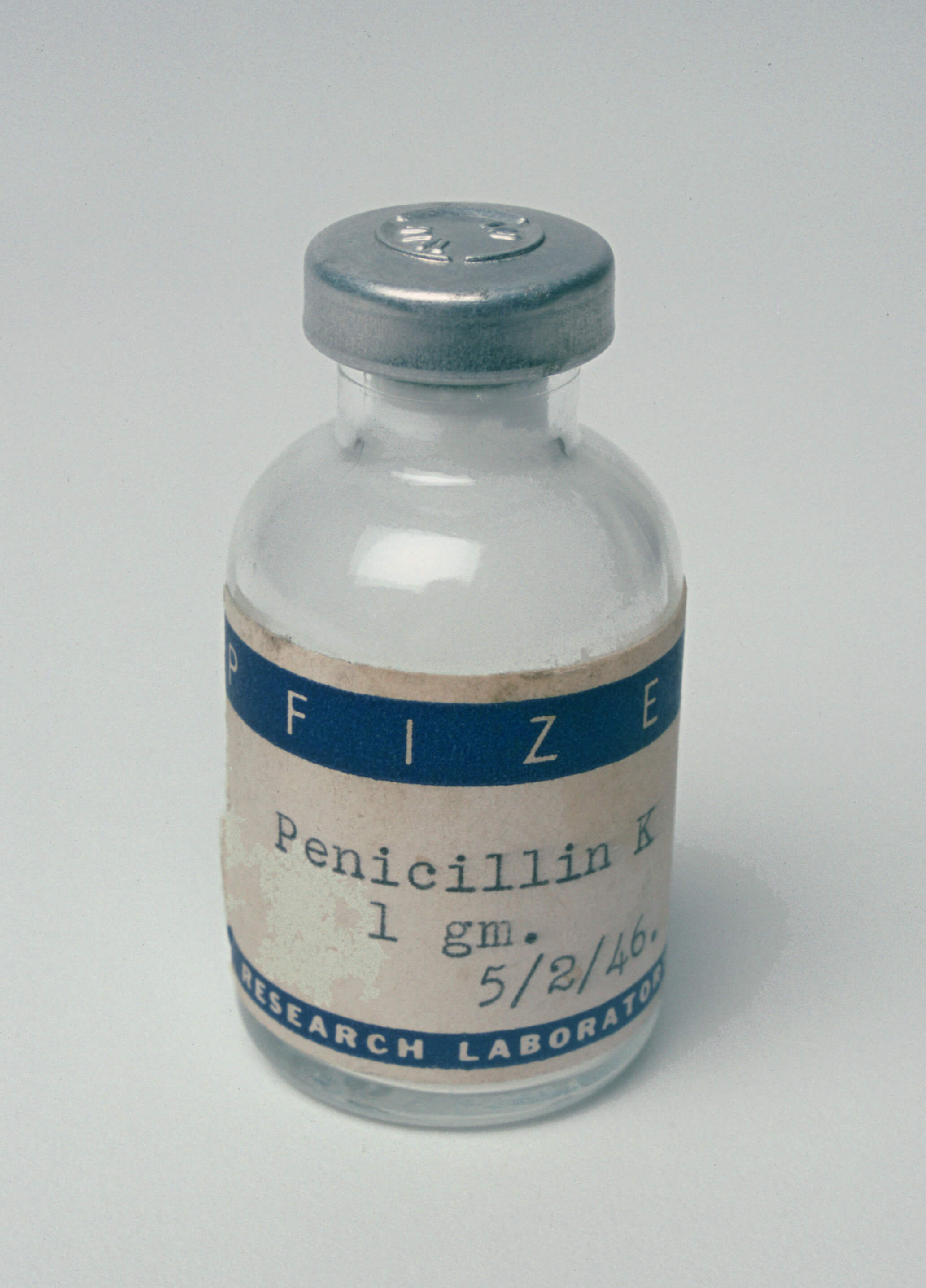

George A. Anderson becomes Pfizer's chairman of the board. John L. Smith fills the office of President.
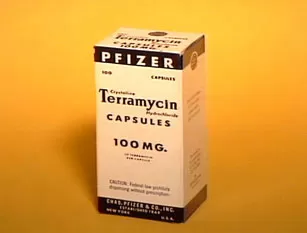
Terramycin® (oxytetracycline), a broad-spectrum antibiotic that is the result of the Company's first discovery program, becomes the first pharmaceutical sold in the United States under the Pfizer label. Pfizer begins expansion into overseas markets and the International Division is created.
Terramycin also marks the beginning of the Pfizer Pharmaceutical Sales Force. Upon its approval by the United States Food and Drug Administration on March 15, 1950, eight specially trained Pfizer pharmaceutical salesmen waiting for word at pay phones across the nation move into action to get inventory to wholesalers and to educate physicians about Pfizer's first proprietary pharmaceutical product. These men are the vanguard of a sales and marketing organization that will come to be recognized as the best in the industry.
Collapse
In a major international expansion, Pfizer operations are established in Belgium, Brazil, Canada, Cuba, England, Mexico, Panama, and Puerto Rico. John "Jack" Powers, Jr., then assistant to Pfizer President John McKeen, directs his international teams to “study the economy, establish proper contacts with government officials, learn the language, history, and customs, and hire local employees wherever possible."
While other companies keep their international employees on a short leash, Pfizer gives its international people tremendous autonomy, enabling them to make critical decisions immediately, rather than waiting weeks, or even months, for the home office to respond. This formula proves to be remarkably successful in the years ahead.
Collapse
Pfizer establishes an Agricultural Division dedicated to offering cutting-edge solutions to animal health problems. The division opens its 700-acre farm and research facility in Terre Haute, Indiana.

After it's acquisition, J.B. Roerig and Company, specialists in nutritional supplements, becomes a division of Pfizer. Roerig remains an integral part of Pfizer's outstanding marketing division.

A fermentation plant opens in England, laying the foundation for Pfizer's research and development operations in Great Britain. Pfizer partners with Japan's Taito to manufacture and distribute antibiotics. Pfizer acquires full ownership of Taito in 1983.

New Pfizer pharmaceutical plants begin production in Mexico, Italy, and Turkey. International personnel increases from 4,300 in 1957 to over 7,000.

The Company signals its increasing commitment to research by consolidating its medical research laboratory operations in Groton, Connecticut.

Pfizer begins a decade of substantial growth and establishes new World Headquarters in midtown Manhattan.

John J. Powers, Jr. President and Chief Executive Officer Chas. Pfizer & Co., Inc. 1966.
John J. Powers, Jr.,is named president and CEO. John McKeen, whom he succeeds, remains chairman of the board, a position he holds until 1968, when Powers assumes full leadership of the company.
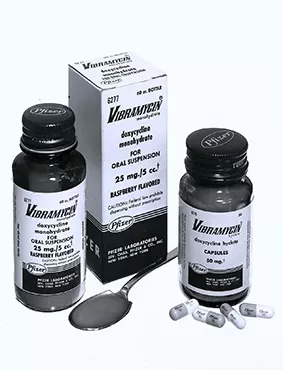
Vibramycin® (doxycycline hyclate), the company's first once-a-day broad-spectrum antibiotic is introduced and quickly becomes a top seller.

Pfizer acquires Mack Illertissen, a prosperous manufacturer of pharmaceutical, chemical, and consumer products oriented to the needs of the German marketplace.The Central Research Division is established, combining pharmaceutical, agricultural, and chemical R&D worldwide. It eventually grows to include research centers on three continents. In an era of unprecedented advances in medical discovery, Pfizer makes a long-term investment in research that will pay off years later.

Pfizer crosses the billion-dollar sales threshold. John Powers, Jr., steps down; Edmund T. Pratt, Jr., becomes CEO; and Gerald D. Laubauch becomes President.

Pfizer introduces Minipress® (prazosin HCI) in the United States, for the control of high blood pressure.
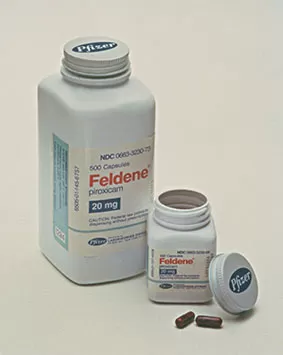
Feldene® (piroxicam) becomes one of the largest-selling prescription anti-inflammatory medications in the world and, ultimately, Pfizer's first product to reach a total of a billion United States dollars in sales.
Glucotrol® (glipizide), for diabetes, is launched.
Pfizer introduces Unasyn® (ampicillin sulbactam), an injectable antibiotic.

The Agricultural Division is renamed the Animal Health Division.
Pfizer launches Procardia® XL (nifedipine) extended-release tablets, an innovative once-a-day medication for angina and hypertension.

William C. Steere, Jr., is appointed President. A year later, he is also named Chief Executive Officer.
Diflucan® (fluconazole), a powerful antifungal, is launched in the United States and 15 additional countries. Originally approved for systemic fungal infections, in 1994 it receives a new indication in the U.S. for vaginal candidiasis. The single-dose Diflucan® tablet is a welcome alternative to the existing treatments that requires topical applications of cream for a week or more.
Collapse
William C. Steere, Jr. becomes Chairman of the Board. His goal is to refocus the Company on its core competencies.
Pfizer has a triple rollout of major new medicines: Zoloft® (sertraline hydrochloride) for treatment of depression, Norvasc® (amlodipine besylate) for control of angina and hypertension, and Zithromax® (azithromycin) for respiratory and skin infections.

Pfizer's Sharing the Care, the industry's premier drug-donation program, is launched. Sharing the Care provides medicines to more than one million eligible low-income and uninsured patients throughout the United States.

The Animal Health Division purchases SmithKline Beecham's animal health business, making Pfizer a world leader in the development and production of pharmaceuticals for livestock and companion animals.
Pfizer increases its presence in the Far East by building a pharmaceutical plant in Dalian, China and expanding throughout growing markets in the Pacific Rim. Cardura® (doxazosin mesylate) is introduced in the United States for the treatment of benign prostate hyperplasia (BPH).
Collapse
Fortune® magazine names Pfizer the world's most admired pharmaceutical company.

Pfizer's roster of outstanding drugs grows with the launch of Viagra® (sildenafil citrate), a breakthrough treatment for erectile dysfunction.
Pfizer invests more than $3.3 billion in research and development.
Pfizer and the Edna McConnell Clark Foundation partner to establish the International Trachoma Initiative (ITI) to help eliminate blinding trachoma. Learn more about Trachoma and the International Trachoma Initiative.
Collapse
Pfizer celebrates its 150th anniversary as one of the world's premier pharmaceutical companies. Recognized for its success in discovering and developing innovative drugs for human discovery, Forbes® magazine names Pfizer "Company of the Year." Pfizer takes the drug discovery process to a new level of efficiency with the opening of the Discovery Technology Center in Cambridge, Massachusetts.
Utilizing the emerging knowledge of gene families, the Center's mission is to evolve new, more efficient models for discovering drug candidates. These candidates have an increased potential to survive the rigors of drug development. Pfizer investment in research and development exceeds $4 billion for the first time. Learn more about Pfizer's commitment to research.
Collapse
The Best Get Better—Pfizer and Warner-Lambert merge to form the new Pfizer, creating the world's fastest-growing major pharmaceutical company. Learn more about the Pfizer/Warner-Lambert merger.
Pfizer and the Ministry of Health of South Africa sign a Memorandum of Understanding to establish the Diflucan® Partnership Program. Learn more about the Diflucan® Partnership Program.
Pfizer opens the largest building in the world dedicated to the discovery of new medicines for human and animal health on its Groton, Connecticut research campus.
Collapse
William C. Steere, Jr. announces his retirement as CEO on January 1, 2001, and steps down as Chairman of the Board in April, following the company's annual meeting. Henry A. McKinnell, Jr., Ph.D. succeeds William C. Steere, Jr. as Chairman and Chief Executive Officer.
In June 2001, Hank McKinnell announces a new mission for Pfizer—to become the world's most valued company to patients, customers, colleagues, investors, business partners, and the communities where we work and live. In July, he announces a commitment to fund the building of a regional treatment and training center on the campus of Makerere University in Kampala, Uganda as part of the Academic Alliance for AIDS Care and Prevention.
Pfizer launches Geodon® (ziprasidone hydrochloride), a new antipsychotic for the treatment of schizophrenia.
Collapse
In a major expansion of its commitment to improving health care for low-income Americans, Pfizer introduces The Pfizer For Living™ Share Card Program. The program provides qualified low-income Medicare beneficiaries with access to up to a 30-day supply of any prescription medicine for a flat rate of $15 per prescription. By April 2004, over half a million seniors enrolled in the program and nearly five million prescriptions were filled.
Pfizer invests an industry leading $5.1 billion in research and development and launches Vfend® (voriconazole), an orally and intravenously administered antifungal indicated for treatment of serious fungal infections.
Pfizer becomes the first U.S. pharmaceutical company and first top-ten company on the New York Stock Exchange to join the U.N. Global Compact, an international network that promotes good corporate citizenship by fostering partnerships between companies, U.N. agencies, non-governmental organizations (NGOs), trade unions and academic institutions.
The Pfizer Foundation announces the launch of a three-year initiative to provide grants to support training and capacity building for HIV/AIDS in developing countries. Twelve organizations receive grants through the International HIV/AIDS Health Literacy Grants Program.
Hank McKinnell, CEO and Chairman of Pfizer, announces the Global Health Fellows program at the World AIDS Conference in Barcelona - a call to action for Pfizer colleagues to volunteer in developing countries for up to six months on HIV/AIDS projects. In 2003, the first eighteen Global Health Fellows are sent into the field.
Collapse
Pfizer invests more than $7.1 billion in research and development.
On April 16, 2003 Pfizer Inc and Pharmacia Corporation combine operations, bringing together two of the world´s fastest-growing and most innovative companies. Learn more about the Pfizer/Pharmacia merger

Pfizer Inc is selected by Dow Jones and Co. to be included in the Dow Jones Industrial Average, which is the best-known stock market barometer in the world.
Caduet® (amlodipine besylate and atorvastatin calcium), the first single pill that treats both high blood pressure and high cholesterol, is launched.
Pfizer Helpful Answers®, the pharmaceutical industry's most comprehensive prescription medicines access initiative is launched, enabling America's 45 million uninsured to obtain Pfizer medicines free or at significant savings.
The Infectious Diseases Institute, a new medical facility providing state of the art training and treatment of HIV/ADS and other infectious diseases, opens its doors on the grounds of Makerere University in Kampala, Uganda. Pfizer Inc and the Pfizer Foundation, as part of a unique public-private partnership with a number of organizations, contribute more than $15 million to support construction of the building.
Collapse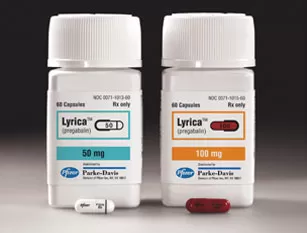
Pfizer launches Lyrica® (pregabalin), the first treatment approved by the U.S. Food and Drug Administration to treat two distinct forms of neuropathic pain associated with diabetic peripheral neuropathy (DPN), postherpetic neuralgia (PHN) and adjunctive treatment of partial onset seizures in adults with epilepsy.
Pfizer's roster of outstanding drugs grows with the launch of Sutent® (sunitinib malate), a new, oral, multikinase inhibitor to treat patients with metastatic renal cell carcinoma (mRCC), or advanced kidney cancer, and gastrointestinal stromal tumor (GIST) after disease progression on, or intolerance to, imatinib mesylate.
Pfizer launches Eraxis™ (anidulafungin), a new medicine to treat certain infections caused by Candida, a yeast-like fungus that can cause serious infections in hospitalized patients or patients with compromised immune systems.
Chantix™ (varenicline), a prescription medicine to help adults stop smoking, is launched by Pfizer.
In July 2006, the Pfizer Board of Directors names Jeffrey B. Kindler Chief Executive Officer. Kindler succeeds Hank McKinnell, who will remain Chairman of the Board until his retirement in February, 2007.
Collapse
Pfizer launches Selzentry™ (maraviroc) tablets, the first in a new class of oral HIV medicines in more than 10 years. Selzentry blocks viral entry into white blood cells, significantly reducing viral load and increasing T-cell counts in treatment-experienced patients infected with a specific type of HIV.
Pfizer launches an online site to provide up-to-date, user-friendly information on the status of its U.S. post-marketing commitments - studies conducted after a medicine receives regulatory approval and designed to provide additional information about the medicine's safety, efficacy or optimal use. This initiative is the first of its kind for a pharmaceutical company.
To help address critical gaps in malaria treatment and education, Pfizer announces the launch of Mobilize Against Malaria.
Collapse
Jeff Kindler, Chairman and CEO of Pfizer, announces the next step in the company's evolution and outlines the company's plan to establish smaller operating units designed to enhance innovation and accountability, while drawing upon the advantages of Pfizer's scale and resources. These customer-focused business units allow Pfizer to better anticipate and respond to customers' and patients' needs, as well responds to changes in the marketplace.
Pfizer launches a new Medicine Safety Website to help healthcare professionals and patients make better informed decisions about treatment options.
Grameen Health, an affiliate of Grameen Bank, the pioneering micro-financing organization in Bangladesh that shared the Nobel Peace Prize in 2006 for its work to alleviate poverty, partners with Pfizer to identify sustainable models for healthcare delivery in the developing world.
Pfizer launches its Global Regenerative Medicine Unit. The unit is dedicated to understanding the biology of stem cells and the opportunity these cells provide, to discover and develop a new generation of regenerative medicines that may prevent disability, repair failing organs and treat degenerative diseases.
Pfizer enters into an agreement with Medivation to develop and commercialize an investigational medicine, Dimebon, for treating Alzheimer's disease and Huntington's disease.
Collapse
On October 15, 2009, Pfizer acquires Wyeth, creating a company with a broad range of products and therapies that touch the lives of patients and consumers every day and at every stage of life.
Pfizer takes a new and unique approach to biomedical research, a move intended bring more innovative medicines to more patients more quickly. Specifically, Pfizer creates two distinct research organizations: The PharmaTherapeutics Research & Development Group, which focuses on discovery of small molecules and related modalities; and The BioTherapeutics Research & Development Group, which focuses on large-molecule research, including vaccines. Learn more about Pfizer's Research and Development organizations.
Pfizer launches Toviaz (fesoterodine fumarate), a prescription medicine used in adults to treat symptoms of a condition called overactive bladder.
Pfizer enters into major licensing agreements with two Indian-based pharmaceutical companies — Claris Lifesciences Ltd. and Aurobindo Pharma Ltd. — to enhance medicinal availability to underserved populations around the world and add new non-Pfizer medicines to the company's existing portfolio of established products.
Because patient participation in clinical trials is the key to progress in medical research, Pfizer enters into a collaboration with Private Access, an innovator in privacy-enhanced search technology, to create a new online community aimed at increasing clinical trial awareness and participation.
Collapse
Ian Read joins Pfizer as Chief Executive Officer. Prior to this, he served as Senior Vice President and Group President of the Worldwide Biopharmaceutical Businesses, which he led from 2006 through December 2010. In that role, he oversaw five global business units—Primary Care, Specialty Care, Oncology, Established Products and Emerging Markets.
Mr. Read began his career with Pfizer in 1978 as an operational auditor. He worked in Latin America through 1995, holding positions including Chief Financial Officer, Pfizer Mexico, and Country Manager, Pfizer Brazil.


Pfizer announces a diversified R&D platform named Pfizer Worldwide Research and Development, supporting excellence in small molecules, large molecules and vaccine research and development.
As part of the acquisition of Wyeth in 2009, Pfizer initially implemented a two-division structure for research and development (BioTherapeutics and PharmaTherapeutics) to ensure the progress and steady integration of both legacy organizations. Due to the speed and effectiveness of that integration, Pfizer progresses to this new model while maintaining the same breadth and research programs. Learn more about Pfizer's Research and Development organization.
Collapse
Pfizer Completes Sale of Capsugel Business to KKR.
Pfizer announces that it has completed the sale of its Capsugel business to an affiliate of Kohlberg Kravis Roberts & Co. L.P. (together with its affiliates, “KKR”), following the receipt of required regulatory clearances, including in the U.S. and the European Union.
Pfizer's subsidiary, Zoetis™ Inc, files IPO registration statement
Pfizer's subsidiary, Zoetis™ Inc., files a registration statement with the U.S. Securities and Exchange Commission for a potential initial public offering (IPO) of Class A common stock. The offering is expected to represent an ownership stake of up to 20 percent. Prior to completion of the offering, which is targeted for the first half of 2013, Pfizer will transfer its animal health business to Zoetis.

Pfizer announces plans to move forward to internally separate its commercial operations into three business segments, two of which will include Innovative business lines and a third which will include the Value business line. Each of the three segments will include developed markets and emerging markets.
The changes are implemented in January 2014 in countries that do not require a consultation with works councils or unions, and are then implemented in countries that require consultation after the successful conclusion of those processes.
Collapse
Pfizer announces that it has entered into a definitive agreement to acquire Baxter International Inc.’s portfolio of marketed vaccines for $635 million. As part of the transaction, Pfizer will also acquire a portion of Baxter’s facility in Orth, Austria, where these vaccines are manufactured.

Pfizer announces that they have entered into a definitive merger agreement under which Pfizer will acquire Hospira. This strategically complementary combination adds a growing revenue stream and a platform for growth for Pfizer’s GEP business.
The expanded portfolio of sterile injectable pharmaceuticals, composed of Hospira’s broad generic sterile injectables product line, including acute care and oncology injectables, with a number of differentiated presentations, as well as its biosimilars portfolio, combined with GEP’s branded sterile injectables, including anti-infectives, anti-inflammatories and cytotoxics, will create a leading global sterile injectables business.
The combination also reinforces GEP’s growth strategy to build a broad portfolio of biosimilars in Pfizer’s therapeutic areas of strength through the addition of Hospira’s portfolio that includes several marketed biosimilars. Pfizer will also use its existing commercial capabilities, global scale, scientific expertise and world class development capabilities to significantly expand the reach of Hospira’s products, which are currently distributed primarily in the United States, to Europe and key emerging markets, where GEP has a significant presence.
Collapse
Watson Health and Pfizer announce a collaboration that will utilize IBM Watson for Drug Discovery to help accelerate Pfizer's research in immuno-oncology, an approach to cancer treatment that uses the body's immune system to help fight cancer.
Pfizer is one of the first organizations worldwide to deploy Watson for Drug Discovery, and the first to customize the cloud-based cognitive tool – tapping in to Watson's machine learning, natural language processing, and other cognitive reasoning technologies to support the identification of new drug targets, combination therapies for study, and patient selection strategies in immuno-oncology.
Immunotherapies, which modify a patient's immune system to recognize and target cancer cells using a combination of vaccines, immunomodulators, and small/large molecules, are reshaping the field of oncology. Oncology researchers at Pfizer will use Watson for Drug Discovery to analyze massive volumes of disparate data sources, including licensed and publicly available data as well as Pfizer's proprietary data. With this new tool, Pfizer researchers will analyze and test hypotheses to generate evidence-based insights for real-time interaction. The customized technology can also support efficient safety assessments.
Collapse
Pfizer and Corning Incorporated announce collaborations that have enabled the modernization of pharmaceutical packaging with the introduction of Corning Valor™ Glass. This revolutionary pharmaceutical glass packaging solution enhances the storage and delivery of today’s drug formulations and provides more reliable access to medicines essential to public health.
Deep pharmaceutical formulation and manufacturing process insights from Merck and Pfizer, in combination with Corning’s glass science and precision forming capabilities helped deliver an exceptional glass packaging solution for injectable drugs in vials and cartridges. The companies’ continued collaborations will focus on additional evaluations and the deployment of this new innovation. Corning Valor Glass packaging offers superior chemical durability, strength and damage resistance. These qualities enable increased throughput and more reliable access to state-of-the-art medicines for patients, while maintaining a high level of quality assurance for pharmaceutical companies.
Collapse
Pfizer leads the world in developing a vaccine and treatment in response to the COVID-19 pandemic and commits to lightspeed manufacturing to expand vaccine and treatment access to people around the world.

Pfizer acquires several companies:
Leadership. Honor. A sense of purpose. The qualities you demonstrated as a member of the armed forces are valued at Pfizer.
We’re turning advanced science and technologies into therapies that make a real difference. Join us, and your efforts could impact millions of people.
We want to create a workplace for all. That’s why we’re committed to increasing diversity and fostering inclusivity in this first-of-its-kind, nine-year fellowship program.
To Our Investors
Our ambitions are big and our product pipeline has never been stronger. We’re energized and inspired to apply science and our global resources to develop and deliver breakthrough therapies to people everywhere.
To Our InvestorsScience & Innovation
In recent decades, advances in breast cancer treatment have helped to improve health outcomes for many patients, but scientists remain vigilant in their quest to further reduce both the incidence and impact of the disease.
Purpose & Ideals
Lyme disease is the most common vector-borne illness in the United States. New research indicates that people of color are more likely to develop more severe Lyme disease.
Science & Innovation
The human gut contains a variety of bacteria and other microorganisms comprising the microbiome, and that’s a good thing! Clostridioides difficile, also known as C. difficile, is one such bacterium.