Metabolism and Immunology: Using Lessons From Metabolic Diseases to Treat Autoimmune Disorders
By regulating the metabolism of immune cells, researchers are uncovering novel ways to suppress inflammation and treat autoimmune diseases.
Scientists have long recognized that metabolic-related disorders such as heart disease, type 2 diabetes and obesity have links to inflammation. But what if the converse were also true? What if inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease had a metabolic component to them?
In recent years, researchers in the burgeoning field of immunometabolism are exploring that exact question. Combining knowledge of cellular metabolism with immunology, experts are studying pathways to alter the metabolism of immune cells to switch off an unwarranted inflammatory response. “We’re working on harnessing the immune system through the control of cellular metabolism and asking whether we can keep the immune system in balance by tuning metabolic pathways,” says Samantha Hiemer, a post-doctoral fellow in the Inflammation and Immunology Unit at Pfizer’s Kendall Square, Cambridge research site.
Inflammation as ‘Friendly Fire’
The immune system is among the most powerful systems in the body, outfitted with an arsenal of defense cells that can destroy pathogens that cross its path. At the same time, the body has a variety of fail-safes to ensure that the immune system doesn’t attack itself. But sometimes these safeguards don’t work, and we get an unwarranted inflammatory response. The simplest way to measure this response is to see a flood of immune cells, such as T-cells and B-cells, migrating into organs and tissues. “Autoimmunity is like friendly-fire – the immune system is too active and is attacking the body it’s supposed to be protecting,” says Hiemer.
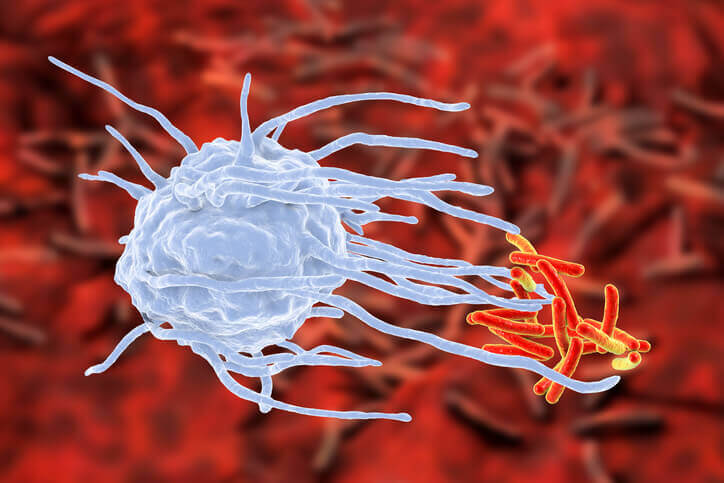
A macrophage shown here engulfing tuberculous bacteria. The immune system is normally in what is called a “quiescent state” — it’s on the lookout for pathogens but not actively in fight mode. When it is time to attack, immune cells start to increase their metabolism, absorbing more nutrients in order produce the proteins essential for defense, such as cytokines and antibodies. The immune system needs to be activated in order for macrophages to be attracted to the bacteria site.
In healthy people, the immune system is normally in what is called a “quiescent state” — it’s on the lookout for pathogens but not actively in fight mode. When it is time to attack, immune cells start to increase their metabolism, absorbing more nutrients in order produce the proteins essential for defense, such as cytokines and antibodies.
One of the check-and-balance mechanisms the immune system uses to regulate a response occurs via its metabolic pathways. The immune system has ways to trick itself into thinking it’s in starvation mode, shutting down metabolism and suppressing an immune response. One example of this is with developing fetuses. Why does the mother’s immune system normally not attack the baby, which contains foreign DNA from its father? The placenta has special cells on its inner lining, called trophoblasts, that release an enzyme known as IDO, tricking the mother’s immune cells inside the womb into perceiving the environment as “nutrient deprived.” “This microenvironment creates a barrier between mother and baby and because the mother’s immune cells don’t have enough nutrients available to mount an immune response, they can’t attack the baby,” says Hiemer.
‘Using a Cannon to Swat a Fly’
Many of the current therapies to treat autoimmune diseases work by suppressing the entire immune system, and are likened to using “a cannon to swat a fly.” While these therapies can successfully block an inflammatory response, they have the unwelcome side effect of leaving patients vulnerable to infection. The hope in developing these new therapies is to slightly alter the metabolism of immune cells to prevent cellular proliferation that causes inflammation while preserving the immune system.

Using immune-suppressing therapies to treat autoimmune disorders can be like “using a cannon to swat a fly.”
Seeking a new therapy to regulate immune cell metabolism, researchers had to look no further than a commonly used diabetes drug that targets mTOR, a metabolic-sensor protein found in virtually all cells. The medicine works by tricking the mTOR protein into thinking there is already enough energy present in the cell and it doesn’t need to be activated. For autoimmune diseases, inhibiting the mTOR pathway can fool cells into thinking they’re in an energy-deprived state, and should not replicate and mount an immune response. “As autoimmune diseases increase in incidence, there is an urgent need for novel therapies that have less side effects. Our research into the interplay between cellular metabolism and immunity is offering an innovative pathway to treat these chronic, debilitating inflammatory conditions,” says Hiemer.
