The People Behind Your Vaccine
Vaccines have played an important role in significantly reducing the global burden of several serious infectious diseases. In fact, according to the World Health Organization (WHO), vaccination is one of the most successful and cost-effective public health interventions available.1 Vaccinations are a medical marvel, but have you stopped to think that behind every syringe is a steadfast, dedicated team—a collective, synchronized effort of people, processes, and resources—all working towards a shared goal: to help protect you, children and adults, across the globe from infectious diseases.
Bringing a vaccine to market is a long and involved journey. At every step, every turn, every test, is one of our passionate employees working tirelessly to produce some of the most complex vaccines currently available.
Read on to experience the journey of vaccine development, meet our talented people and see what makes this global team so special. Join the conversation on LinkedIn at https://www.linkedin.com/company/pfizer/
The Process and People Behind Your Vaccine
Bringing a vaccine to market is a complex global effort that requires input from various expertise—including research and development, manufacturing, quality control, production, supply chain, and communications.
Vaccine Research and Development
The long journey of vaccine development begins at the laboratory, where scientists research how best to protect against dangerous germs and undergo years of lab tests on a vaccine candidate. If these lab results are promising, volunteers are then enrolled to participate in carefully monitored clinical trials.2 The vaccine candidate goes through several phases of clinical trials, which can take years to complete. Once the vaccine candidate has passed the research and clinical trials phases, the country’s governing body reviews the data and decides whether or not to license the vaccine.3
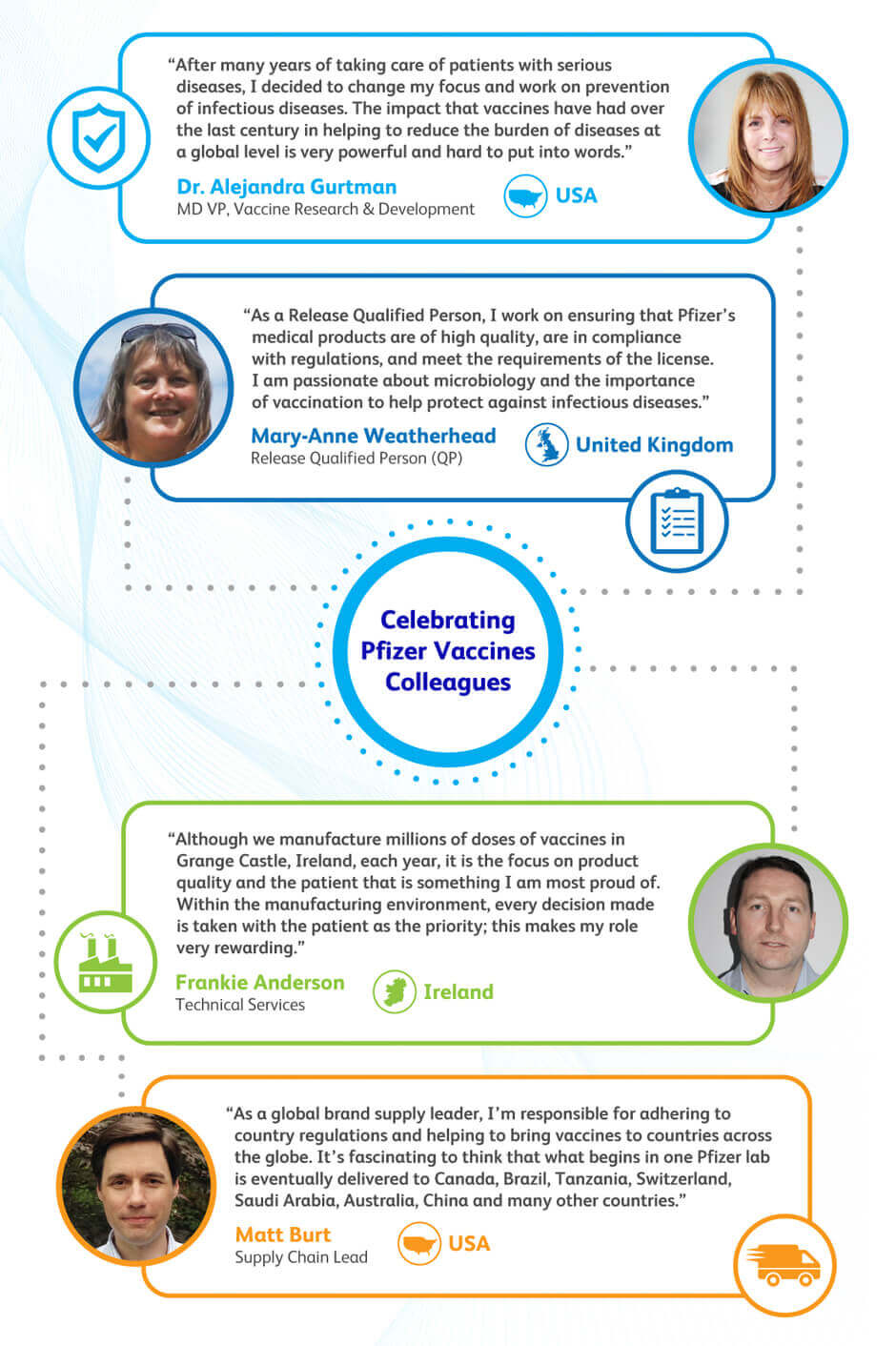
Pfizer’s research teams work day in and out to bring innovation to the vaccines space and address unmet medical needs. Vaccine Research and Development Vice President, Dr. Alejandra Gurtman, explains “After many years of taking care of very sick patients, I decided to change my focus and work on prevention. The impact that vaccines have had over the last century in helping eliminate or reduce the burden of serious infectious diseases at a global level is very powerful and hard to put into words.”
Manufacturing and Quality Control
Manufacturing can be a lengthy process that requires the coordination of many people and steps. An integral part of this coordinated effort is ensuring that all products meet the necessary regulatory and compliance requirements. Mary-Anne Weatherhead, a Release Qualified Person (QP) in the United Kingdom, shares her experience, “As a Release Qualified Person, I work on ensuring that Pfizer’s medical products are of high quality, are in compliance with regulations, and meet the requirements of the license.”
During and after the manufacturing process, vaccines are continuously regulated for quality control. Frankie Anderson, who leads the Vaccines Technical Services team at the Pfizer Grange Castle site in Ireland, explains “Although we manufacture millions of doses of vaccines in Grange Castle, Ireland, each year, it is the focus on product quality and the patient that is something I am most proud of. Within the manufacturing environment, every decision made is taken with the patient as the priority; this makes my role very rewarding.”
Supply Chain and Dissemination
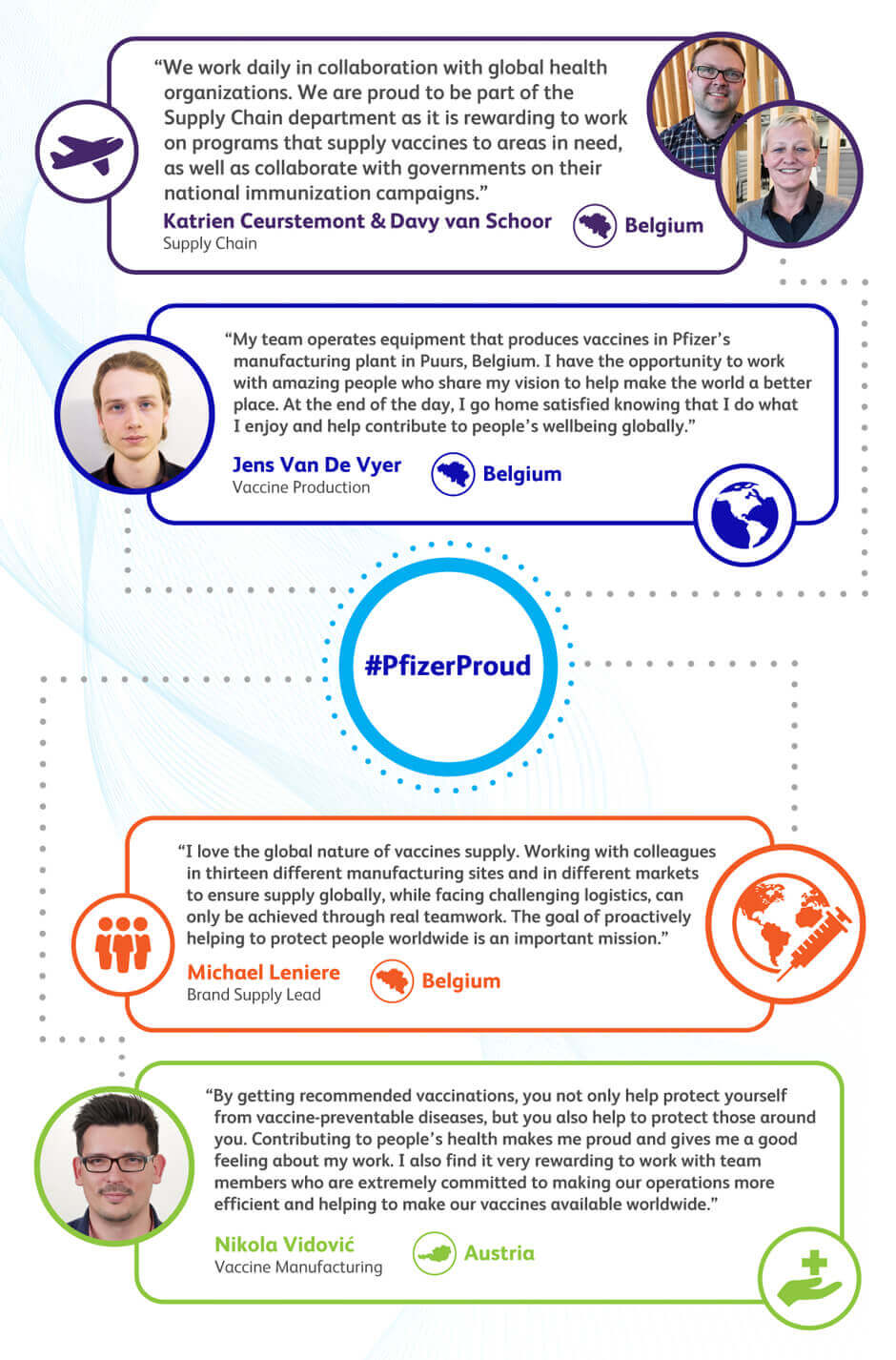
Supply Chain is integral in ensuring vaccines are delivered on time, safely, and in the correct quantity worldwide. Global supply chain lead, Matt Burt, explains that because Pfizer provides its products globally, he is “responsible for adhering to country regulations and helping to bring vaccines to countries across the globe.” He demonstrates the global reach and connectivity of Pfizer vaccines, stating “It’s fascinating to think that what begins in one Pfizer lab is eventually delivered to Canada, Brazil, Tanzania, Switzerland, Saudi Arabia, Australia, China and many other countries.”
To increase access of Pfizer vaccines in lower income countries and emerging markets, Pfizer works closely with global health leaders and governments on immunization programs, vaccine delivery and public health programs. For example, Katrien Ceurstemont and Davy van Schoor, of the Supply Chain Department in Belgium, work closely with global health organizations to supply vaccines and support national immunization programs, such as working with the Bhutan government to support their national immunization campaign. Katrien and Davy are “proud to be part of the Supply Chain department as it is rewarding to work on programs that supply vaccines to areas in need, as well as collaborate with governments on their national immunization campaigns.”
Pfizer Vaccines is infusing the power of transformative science into disease prevention. Our colleagues are committed to pursuing research and forming partnerships that help speed the development and delivery of new, life-saving vaccines.4
Vaccines have made major improvements in global public health by greatly reducing the burden of several serious infectious diseases worldwide. However, none of this would be possible without the talent, commitment and passion of our colleagues. Thank you, all!
1World Health Organization (WHO). 10 Facts on Immunization. Updated March 2018. https://www.who.int/features/factfiles/immunization/en/. April 9, 2019.
2Centers for Disease Control and Prevention (CDC). The Journey of Your Child’s Vaccine. Updated on January 26, 2018. https://www.cdc.gov/vaccines/parents/infographics/journey-of-child-vaccine.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fparents%2Finfographics%2Fjourney-of-child-vaccine-text.html. April 18, 2019.
3U.S. Food and Drug Administration (FDA). Vaccine Product Approval Process. Updated on February 2, 2018. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/vaccine-product-approval-process. April 18, 2019.
4Pfizer 2017 Annual Review. Vaccines. https://www.pfizer.com/files/investors/financial_reports/annual_reports/2017/our-innovation/vaccines/index.html. Accessed April 2019.
