CEO Letter
2017: Building on Our Strengths
In 2017, we continued to drive growth in many of our anchor brands, received a record number of product approvals, made significant advances in our R&D pipeline, and further strengthened our hallmark ownership culture.
To Our Shareholders:
Every day, more than 90,000 Pfizer colleagues come to work with a singular purpose: to innovate to bring therapies to patients that significantly improve their lives. This unwavering focus on patients is at the core of who we are as a company. It drives the discovery of life-changing innovations. It fuels our passion for improving global health. It encourages collaboration, thoughtful risk-taking and diversity of thought. And in 2017, it enabled us to build on our already formidable strengths – world-class science, industry-leading products, prudent capital allocation, and our unique OWNIT! culture – to deliver another strong year for our company and position us to capitalize on exciting new growth opportunities in 2018 and beyond.
Our Global Footprint
(as of December 31, 2017)
>90,000
employees
>125
countries where we sell our products
58
manufacturing sites worldwide
Strong Fundamentals Drove Solid Financial Performance
Despite an approximately $3.2 billion negative revenue impact due to the loss of exclusivity of certain brands and the divestment of Hospira Infusion Systems, we reported flat operational revenue for 2017 thanks to growth in many of the biggest selling medicines in our portfolio, including Ibrance, Eliquis and Xeljanz – all of which currently have market-leading positions and many years of patent protection remaining. We saw continued growth in emerging markets in 2017, which was up 11 percent operationally compared with the previous year, and in our biosimilars business, which grew 66 percent operationally. We remain the No. 1 biosimilars company globally and have taken steps to fortify that leadership, advancing six biosimilar pipeline products during the year through various regulatory and data milestones. We expanded our portfolios for sterile injectables (28 product launches in 2017) and anti-infectives (launching Zavicefta in more than 15 countries and acquiring the rights to Cresemba in Europe, China and APAC). We also have worked diligently toward remediating issues in the legacy-Hospira manufacturing plants and reducing the related product shortages.
Harnessing the Power of Science to Create Value
One of our most important accomplishments in 2017 was the continued strengthening of our R&D pipeline, which today is as strong as it’s ever been. We have sharpened our focus; we are making better, quicker decisions; and we are accelerating the time it takes to get newly approved products in the hands of patients. We advanced 43 assets in our pipeline and received 10 approvals from the FDA – significantly more than we had achieved in any year in the past decade. Over the next five years, we see the potential for approximately 25-30 approvals, of which up to 15 have the potential to be blockbusters – subject to some expected attrition. This presents an unprecedented opportunity to have a life-changing impact on a growing number of patients while creating enhanced value for all of our stakeholders.
Imperative 1: Ensure a productive, industry-leading innovative core
In 2017:
10 product approvals
8 regulatory submissions
43 pipeline assets advanced
Since our imperatives launched in 2011:
- Strengthened our decision-making process
- Sharpened our focus to five therapeutic areas
- Continued to improve productivity and efficiency
- Secured 30 product approvals – including five products approved with blockbuster potential
Allocating Capital to Benefit Patients and Shareholders
We continue to make capital allocation decisions that maximize benefits for patients and enhance shareholder value. We strongly believe that over the next five years, the biopharmaceutical companies that generate meaningful value for patients in terms of health and quality of life and for the healthcare system in terms of cost/benefit will be the ones that thrive. As patient value becomes the critical determinant of access, price and utilization, the interests of patients become even more strongly aligned with the interests of shareholders. Helping as many patients as possible with breakthrough medicines that improve therapeutic outcomes is inextricably linked to our future revenue growth potential. In 2017, we continued to invest in our business – including funding the discovery and development of potentially life-changing treatments – while simultaneously returning $12.7 billion directly to shareholders through a combination of dividends and share repurchases.
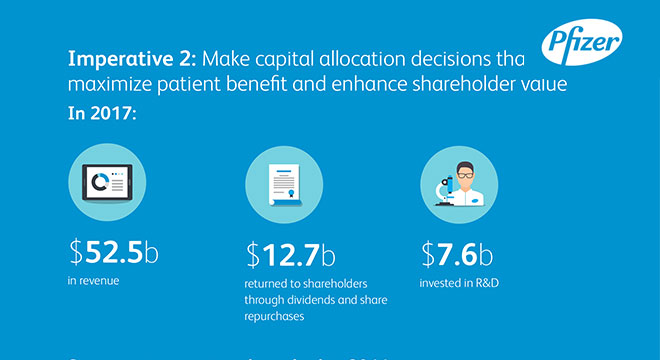
Imperative 2: Make capital allocation decisions that maximize patient benefit and enhance shareholder value
In 2017:
$52.5b in revenue
$12.7b returned to shareholders through dividends and share repurchases
$7.7b invested in R&D
Since our imperatives launched in 2011:
- Returned approx. $110 billion directly to shareholders through a combination of dividends and share repurchases (2010-2017)
- Increased our stock price by 97 percent – or 151 percent, inclusive of dividends reinvested (Reflects change in stock price from Feb. 11, 2011 – Jan. 31, 2018)
Partnering to Improve Global Health
At Pfizer, we recognize that it’s not enough to discover, develop and bring life-saving medicines and vaccines to market if the people who need these treatments can’t get access to them. In 2017, we continued to work to support affordable access to healthcare through partnerships like Access Accelerated, which is working to improve access to treatment and care for non-communicable diseases (NCDs) in low- and middle-income countries; through our new collaboration with the American Cancer Society and the Clinton Health Access Initiative to expand access to essential cancer treatment medications in six sub-Saharan African countries; and by actively engaging with industry groups like the European Federation of Pharmaceutical Industries and Associations (EFPIA), the Pharmaceutical Research and Manufacturers of America (PhRMA) and others to advocate for policies that support affordable access, incentives for investments in science and innovation, and rebate reform.
Imperative 3: Be a responsible corporate citizen
In 2017:
16m+ Shipped 16+ million units of Sayana® Press (medroxyprogesterone acetate) to 23 countries in the developing world, potentially reaching more than 4 million women
1m+ Committed to donate up to 1 million doses of Naxolene Hydrochloride Injection, USP to Direct Relief over four years (up to 250,000 doses per year), and $1 million in opioid overdose grants to five states (Illinois, Massachusetts, new Mexico, New York and Tennessee)
$2.75m The Pfizer Foundation1 donated $2.75 million in cash grants globally to disaster relief and response
30,000 Hundreds of colleagues volunteered to pack approximately 30,000 relief kits
2,200 generators provided to colleagues in Puerto Rico following Hurricane Maria
Since our imperatives launched in 2011:
- Helped more than 2.2 million patients receive over 29.3 million Pfizer prescriptions for free or at savings2
- Matched $74,147,751.85 in colleague donations through Pfizer Foundation Matching Gifts
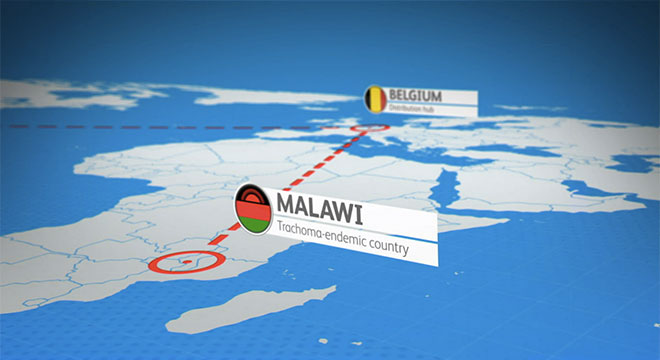
- Donated 465 million doses of the antibiotic Zithromax® to support the International Trachoma Initiative
- The Pfizer Foundation is a charitable organization established by Pfizer Inc. It is a separate legal entity from Pfizer Inc. with distinct legal restrictions.
- Data on file. The Pfizer patient assistance program is a joint program of Pfizer Inc. and the Pfizer Patient Assistance Foundation™. The Pfizer Patient Assistance Foundation is a separate legal entity from Pfizer Inc. with distinct legal restrictions.
Our Four Imperatives
While our purpose has brought focus to our company’s journey, our Four Imperatives have provided us with a clear roadmap to follow. Since being introduced globally in February 2011, they have represented the critical areas that need to be addressed if we were going to ensure Pfizer’s future success: innovation, capital allocation, corporate responsibility and culture. I invite you to click on the four video links accompanying this letter to learn more about our imperatives and the transformational impact they continue to have on our company.
Imperative 4: Continually strengthen our ownership culture
In 2017:
Achieved 85% employee engagement score – among the highest in our industry*
20%+ 20+ percent of our workforce – 21,000+ colleagues – actively engaged in a Colleague Resource Group
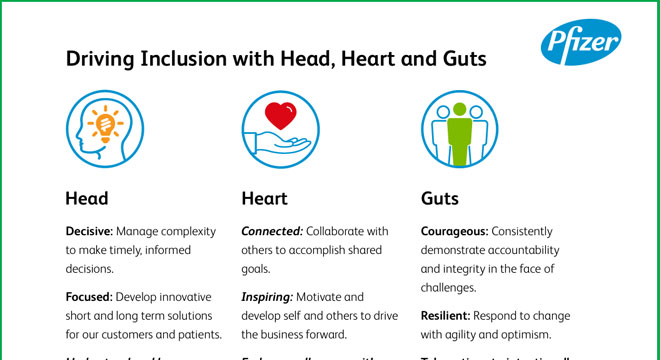
Launched the Head, Heart and Guts leadership initiative, a simple, memorable way for colleagues to demonstrate leadership, regardless of role or level
Amplified our OWNIT! culture through increased honest and meaningful discussions among colleagues and an environment that empowers them to be decisive in addressing the complex issues
Since our imperatives launched in 2011, we have seen:*
- 28 percent improvement in confronting jerk-like behaviors in the workplace
- 14 percent improvement in encouraging thoughtful risk taking
- 7 percent improvement in acting with speed and decisiveness
* Percentages based on the company's annual colleague engagement survey results
Here at Pfizer, every clinical trial, every product launch and every patient served brings us one step closer to becoming the premier innovative biopharmaceutical company in the world. Thank you for your continued support of the complex, important and fulfilling work we do every day.

Ian C. Read
Chairman and CEO
* We encourage you to read our 2017 Financial Report, which includes our financial statements as of and for the year ended December 31, 2017. Please also refer to our Annual Report on Form 10-K for the year ended December 31, 2017, including the sections captioned “Risk Factors” and “Forward-Looking Information and Factors that May Affect Future Results,” for a description of the substantial risks and uncertainties related to the forward-looking statements included herein.