Our business, our purpose
Global Businesses – PIH
At Pfizer Innovative Health (PIH), putting the patient first defines how we organize ourselves and evaluate performance across our six business groups – Consumer Healthcare, Inflammation & Immunology, Internal Medicine (cardiovascular and metabolic diseases and pain), Oncology, Rare Disease and Vaccines – and, most of all, it keeps us committed to creating a healthier world.
Our Emerging Markets group is dedicated to bringing innovative medicines and vaccines to the people and patients that need them across Latin America, Africa, the Middle East and emerging Asia.
As a team, we are committed to using science to change the outcome of chronic conditions and devastating diseases around the world. We are focused on improving health with our innovative medicines and vaccines – from prevention to treatment to wellness – at every stage of life in communities across the globe. Everything we do is to help ensure that people have a chance to live longer, get stronger and lead more vibrant lives.
Consumer Healthcare
In addition to our broad portfolio of prescription medicines, Pfizer is one of the largest over-the-counter (OTC) healthcare companies in the world. Our non-prescription medicines for pain management, respiratory and digestive health, as well as our nutritional and personal care products, are available in more than 90 countries. We market 10 consumer healthcare brands with more than $100 million in annual sales in 2017, and two of the top 10 global OTC brands – Advil®, the world’s number one analgesic brand, and Centrum®, the world’s top-selling multivitamin.
Growing Our Consumer Portfolio
Inspired by consumer insights, Pfizer continually enhances its products to meet evolving customer needs and preferences. For instance, in 2017 we added gummy forms to our Emergen-C® family of products and launched new Emergen-C Energy+ and Emergen-C Hydration+ sport drink mixes. We also expanded our Centrum® MultiGummies® offerings in the U.S., introducing Centrum® MultiGummies® Multi + Omega-3, a multivitamin formulated with nutrients for heart, brain and eye health, and Centrum® MultiGummies® Multi + Beauty, with biotin and other nutrients to support a beautiful body plus hair, skin and nails. We also launched Nexium® 24HR ClearMinis™, meeting consumer needs for frequent heartburn relief in easy-to-swallow capsules, and Advil® Liqui-Gels® minis, which deliver fast, powerful pain relief, at one-third the size of normal Liqui-Gels®.
Bringing Our Products to Consumers in Need
Pfizer has a proud heritage of supporting U.S. troops at home and abroad. During the 2017 holiday season, we donated our consumer products to Operation Gratitude and the USO to support U.S. troops deployed overseas. In 2017, we donated more than 540,000 ChapStick®, Advil®, Robitussin®, Emergen-C® and other products to organizations such as the American Red Cross, Americares and Direct Relief International.
Empowering People to Take Health and Wellness into Their Own Hands
In 2017, Pfizer launched an ambitious, digital content partnership in the U.S. with Meredith Corporation. Online articles, videos, listicles and other content featured in leading consumer publications, such as Better Homes & Gardens, Eating Well, Shape, Martha Stewart Living and Parents, reached millions of consumers to help them take health and wellness into their own hands.
Similarly, to advance understanding of how self-care, aging and technology are impacting trends in health and wellness, we hosted a panel discussion at the International Association of Gerontology and Geriatrics World Congress. Participants examined the impact of aging on consumer behavior and the need for investments in health care technologies, such as e-labeling and mobile medical apps, as well as how the conversion of prescription medicines to OTC availability could potentially benefit consumers who are 60 years of age and older.
Evaluating Our Consumer Healthcare Portfolio
Pfizer announced in October that it is reviewing strategic alternatives for its Consumer Healthcare business. A range of options are being considered, including a full or partial separation of the Consumer Healthcare business from Pfizer through a spin-off, sale or other transaction. Pfizer may ultimately determine to retain the business. This review of strategic alternatives is part of Pfizer’s continuing efforts to allocate resources and capital to best serve patients and maximize value for its shareholders. Pfizer expects that any decision regarding strategic alternatives for Pfizer Consumer Healthcare would be made during 2018.
Inflammation & Immunology (I&I)
Pfizer is a leader in applying cutting-edge science to help transform the lives of people with inflammatory and autoimmune conditions. Over the last several decades, the treatment paradigms for these diseases have expanded from steroids to biologics to novel treatment classes across disease areas to immuno-modulation and precision medicine. We have been a catalyst in this progress, consistently developing and delivering innovative medicines and bringing multiple I&I therapies to market. Today, we are focused on advancing the standard of care in three areas: rheumatology, medical dermatology and gastroenterology. At Pfizer I&I, we also go beyond medicine to truly put our patients-first mission into action by engaging more closely with the communities we serve.
Striving to Address Patient Needs in Rheumatology
Pfizer has been committed to improving patients’ lives in rheumatology for more than 60 years, including the introduction of Xeljanz® (tofacitinib) for moderate-to-severe active rheumatoid arthritis (RA) in 2012, and Xeljanz® XR (tofacitinib) in 2016. The Xeljanz® extended-release formulation is designed to deliver the drug at a slower release rate than Xeljanz twice daily, allowing for once-daily dosing. With additional approvals in 2017 in the EU and China, Xeljanz® is now approved in more than 80 countries and has been prescribed to more than 100,000 patients worldwide. In 2017, global sales for Xeljanz® reached $1 billion.
Building on Pfizer’s heritage in rheumatology, in December we announced the approval of Xeljanz and Xeljanz XR for people with active psoriatic arthritis (PsA) in the U.S. Psoriatic arthritis is a complex, progressive and, at times, debilitating disease with an unpredictable course, and the approval of Xeljanz® in PsA is an important step forward for patients seeking new treatments.
Going beyond medicines, Pfizer has been working to address difficult-to-tackle topics that the RA community has identified, including intimacy, relationships and communication. In 2017, we hosted our second ReAL Talk Summit, bringing together patient advocates and experts to discuss these often taboo topics. Many of the insights from the ReAL Talk Summit are brought to the broader community through Arthritis.com, an online resource where people impacted by arthritis can find inspiration, advice, tools and disease information.
Advancing Treatments in Gastroenterology
In July, the U.S. Food and Drug Administration (FDA) initiated review of our supplemental New Drug Application (sNDA) for Xeljanz for the treatment of moderately to severely active ulcerative colitis (UC). The Prescription Drug User Fee Act (PDUFA) action date for Xeljanz in UC is June 2018. The European Medicines Agency (EMA) also initiated review of Xeljanz in UC in August. If approved by the FDA, Xeljanz would be the first oral Janus kinase (JAK) inhibitor available as a therapeutic option for people living with moderately to severely active UC.
Making a Difference for People Living with Eczema
In early 2017, Eucrisa® (crisaborole) was launched in the U.S., bringing the first new topical treatment option in more than a decade to adults and children living with mild-to-moderate eczema, the most common form of eczema. Eucrisa is steroid-free and can be used on all skin tones, and almost everywhere on the body, for patients as young as two years of age. Global regulatory submissions are also currently being evaluated for Eucrisa.
Internal Medicine
In Internal Medicine, Pfizer's mission is to drive innovation that prevents, diagnoses and treats the most prevalent diseases facing our society. Our portfolio of leading medicines treats common and debilitating health problems faced by today’s largest patient populations, such as heart disease, stroke, smoking and pain. Internal Medicine’s early discovery efforts focus on underserved areas in cardiovascular and metabolic diseases and pain. With dedicated research teams based in a life sciences hub in Cambridge, Massachusetts, we are forging ahead with novel approaches and technologies seeking to address some of the most complex and devastating diseases of our time, including non-alcoholic steatohepatitis (NASH) and chronic pain.
Cardiovascular and Metabolic Diseases
Cardiovascular diseases (CVDs) remain the leading cause of global mortality, accounting for one in every two adult deaths worldwide. The rates of CVD-related morbidity, including heart failure, peripheral arterial disease and nephropathy, are increasing as more patients survive hearts attacks and the population ages. In addition, metabolic diseases, specifically type 2 diabetes and obesity, are major health problems that have reached epidemic proportions worldwide. We are dedicated to developing therapies to treat, slow or prevent disease progression and improve the quality of life for patients with these diseases.
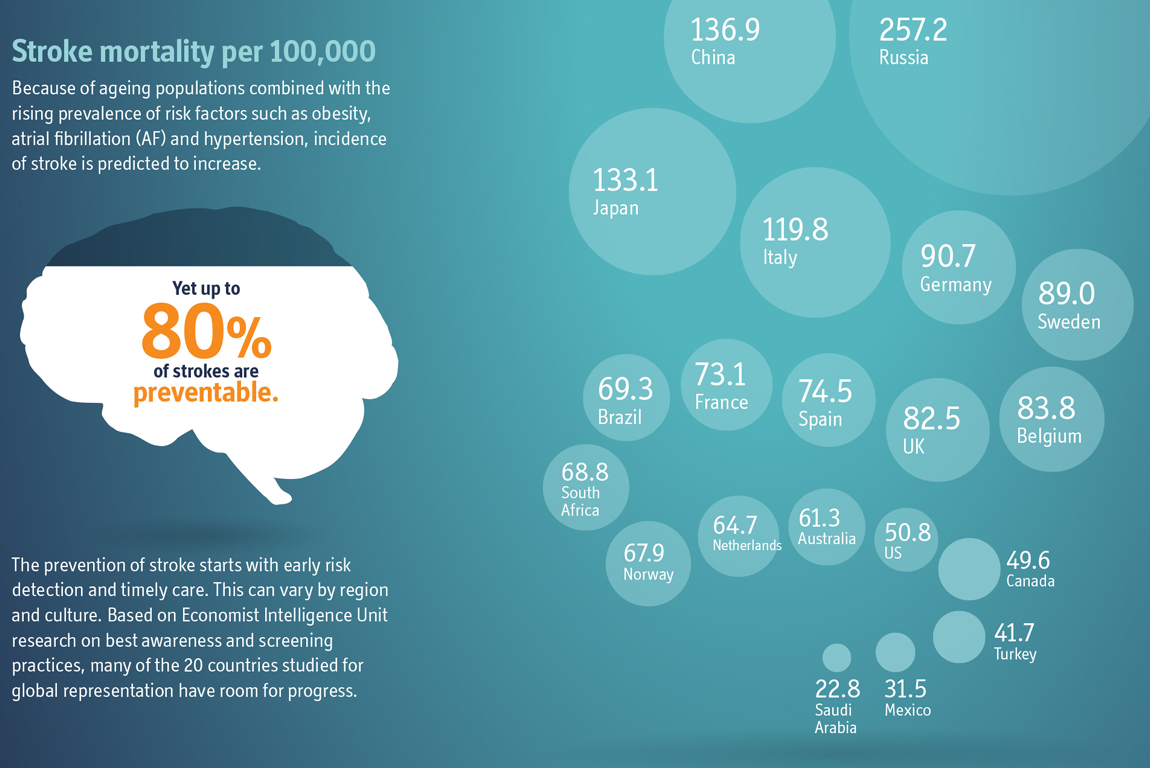
Highlighting the Need for Increased Atrial Fibrillation Screening
Atrial fibrillation (AFib) is a common risk factor for stroke and, despite well-established risks, an estimated one out of three patients with AFib remains undiagnosed. Together with Alliance partner Bristol-Myers Squibb (BMS), Pfizer sponsored Preventing Stroke: Uneven Progress, a research report by the Economist Intelligence Unit, a division of The Economist and a leader in global business intelligence, to assess needs and opportunities for reducing risks of stroke. Based on an analysis of 20 countries, the report found that more than 75 percent of people 65 years of age and older are not screened for AFib and other common stroke risk factors during routine primary care examinations. We are committed to helping overcome the challenges of AFib diagnosis and detection, with the goal of ultimately decreasing the incidence of stroke. As part of this commitment, we are collaborating with partners around the globe on a variety of initiatives to better understand the scale and scope of challenges in preventing stroke.
Real-World Data Analyses and Clinical Data for Eliquis
Eliquis® (apixaban), Pfizer’s oral anticoagulant developed and commercialized in collaboration with BMS, has continued to generate data regarding its use through our clinical trial program and analyses from our real-world data program. The Phase 4 EMANATE clinical trial measured the occurrence of acute stroke, systemic embolism, major bleeding, clinically relevant non-major bleeding and all-cause death in non-valvular atrial fibrillation patients undergoing cardioversion. Analyses from our real-world data program, ACROPOLIS™, focus on the use of Eliquis in routine clinical practice, including among patients considered at high risk of stroke or major bleeding due to age, risk prediction scores and other cardiovascular comorbidities. The results of these studies and analyses continue to grow the body of evidence about Eliquis and further our understanding of the patients who may benefit from treatment.
Pursuing Breakthroughs in the Treatment of NASH
Non-alcoholic steatohepatitis (NASH) is a serious, progressive liver disease that affects an estimated three to five percent of the global population.
Currently there are no approved medicines for NASH. The disease is expected to be the leading cause of liver transplants within the next decade. A critical first step toward successful NASH treatment will be improving diagnostics. Pfizer is working collaboratively with many stakeholders to explore possible diagnostic tools that address the critical need for non-invasive biomarkers and imaging technologies to replace the cumbersome and expensive liver biopsy currently needed to confirm diagnosis.
We are taking a comprehensive, data-driven, multi-pathway approach that explores different and potentially complementary mechanisms of action in NASH. Three assets are in clinical development, and several potentially first-in-class pre-clinical candidates are under investigation. Our objective is to treat NASH by reducing excessive fat content and inflammation in the liver and ultimately reversing scar tissue formation (or fibrosis) in the liver.
Pain
Global estimates suggest that 20 percent of adults suffer from pain and 10 percent are newly diagnosed with chronic pain each year. In the U.S., about 100 million adults – one in three – suffer from chronic pain. Three main types of pathophysiology can be considered to result in pain in the majority of patients: nociceptive pain, neuropathic pain and sensory hypersensitivity. It is important to recognize that multiple pain conditions may coexist, and chronic pain may change over time.
Developing Novel Therapies for Chronic Pain
Pfizer and Eli Lilly and Company are co-developing tanezuamb, an investigational chronic pain treatment being studied for osteoarthritis, chronic low back pain and cancer pain. It is estimated that there are more than 27 million people in the U.S. currently living with osteoarthritis and 23 million living with chronic low back pain. Many of these people are not able to achieve adequate pain relief despite treatment with various types of pain medications. If approved, tanezumab would be the first in a new class of non-opioid chronic pain medications known as nerve growth factor (NGF) inhibitors. By inhibiting NGF, tanezumab may help to keep pain signals produced by muscles, skin and organs from reaching the spinal cord and brain. Tanezumab has a novel mechanism of action that acts in a different manner than opioids and other analgesics, including nonsteroidal anti-inflammatory drugs (e.g., ibuprofen and acetaminophen). Importantly, there is no clinical or non-clinical evidence to indicate that inhibition of NGF would result in addiction, dependence or abuse.
Accelerating Research in Pain Management
The National Institutes of Health (NIH) plays a vital role in the U.S. scientific and biomedical ecosystem. Pfizer advocated for the 21st Century Cures Act, which provided for increased NIH funding, and we applaud the implementation of Section 1003, which establishes an account for the State Response to the Opioid Abuse Crisis. We also joined the NIH in a new public-private partnership designed to accelerate research of novel pain therapies. Through this partnership we can leverage our broad scientific expertise in pain therapeutics and focus on accelerating the development of new, non-opioid pain medications. The NIH Industry Partnership focuses on:
- Accelerating the development of non-opioid analgesics to manage chronic pain;
- Developing therapies to counteract and reverse overdosing;
- Discovering new, innovative medicines to treat opioid addiction, including the use of non-opioid addiction and overdose therapies;
- Leveraging precision medicine approaches to identify genetic variants that are associated with addiction behaviors; and
- Developing biomarkers that help guide clinical trial development and speed up development of new therapies.
Continuing to Provide Benefit with Lyrica®
In October, the FDA approved Lyrica® CR (pregabalin) extended release tablets CV as once-daily therapy for the management of neuropathic pain associated with diabetic peripheral neuropathy and the management of postherpetic neuralgia.
Lyrica CR offers a new treatment option for patients managing these often-debilitating pain conditions, with the convenience of once-daily dosing.
Global Stroke Prevention Policies
Oncology

Pfizer’s advances in oncology exemplify the power of scientific innovation to transform lives. From immuno-oncology to cell therapy to the continued evolution of personalized medicine, our portfolio and pipeline of cutting-edge therapies aim to change the way the world approaches cancer treatment. We apply that same rigor to meeting the day-to-day emotional, financial and educational needs of these patients in order to help improve their lives.
In 2017, Pfizer Oncology achieved a large number of product approvals by the FDA, EMA and regulatory authorities around the globe. These approvals included Bavencio® (avelumab) - in partnership with EMD Serono, the biopharmaceutical business of Merck KGaA - an immuno-oncology agent and the first approved therapy for the treatment of metastatic Merkel cell carcinoma, a rare and aggressive skin cancer, as well as for the treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy, Besponsa™ (inotuzumab ozogamicin) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), and Mylotarg™ (gemtuzumab ozogamicin) for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia (AML) or for patients two years of age and older with CD33-positive AML who have experienced a relapse or who have not responded to initial treatment (refractory), as well as expanded indications for Ibrance® (palbociclib) in metastatic breast cancer, Sutent® (sunitinib malate) for adjuvant treatment of renal cell carcinoma, and Bosulif® (bosutinib) in newly-diagnosed chronic phase (CP) Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia.
Mindful that patients need support in managing their life with cancer, we worked closely with the professional and advocacy communities to develop and expand programs like Pfizer Oncology Together™, a one-stop shop for patients to get resources related to our therapies, and This Is Living With Cancer™, which helps provide additional tools for cancer patients, their families and caregivers.
Rare Disease
Through the application of our science, knowledge and expertise, we can help make a significant impact on the lives of patients suffering from rare diseases and address their unmet medical needs. Our focus on rare disease builds on more than two decades of experience, a dedicated research unit focusing on rare disease and a global portfolio of pipeline and marketed medicines within several disease areas that include hemophilia, sickle cell disease, amyloidosis, neuromuscular and inherited metabolic disorders.
The science behind our work in rare disease combines pioneering clinical research and a deep understanding of how diseases work, with insights from strategic collaborations with academic researchers, patients and other companies to deliver transformative treatments and solutions.
One area where Pfizer sees incredible potential is in gene therapy. We have been working for many years to explore the promise of gene therapy by leveraging our expertise and through strategic investments, including manufacturing facilities, new partnerships and collaborations. In 2017, we invested $100 million in a facility in North Carolina to support manufacturing of innovative gene therapies, building on our 2016 acquisition of Bamboo Therapeutics, Inc., a biotechnology company focused on gene therapy. We also signed an exclusive, global collaboration and license agreement with California-based Sangamo Therapeutics, Inc. for the development and commercialization of gene therapy programs for hemophilia A.
Vaccines

Pfizer has a rich history in vaccine research and development, and the significant impact of our vaccines dates back more than a century. Today, our mission is to “protect lives with innovative vaccines to fight serious disease worldwide,” and Pfizer Vaccines employees are helping people around the globe live healthier lives.
A major milestone in 2017 was the approval for Trumenba® (Meningococcal Group B Vaccine) in Europe and Australia for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroup B (MenB) in individuals 10 years of age and older, as well as in Canada for individuals 10 through 25 years of age. This was followed by seven subsequent launches in the U.K., Malta, Germany, Denmark, Norway, Finland and Portugal. The broader availability of Trumenba reinforces our dedication to advancing novel vaccines that can help protect adolescents and young adults, a population at an increased risk for meningococcal disease.
Our vaccine research and development program focuses on the life continuum – spanning pediatric, adolescent and adult health. We are working to develop a vaccine to help protect newborns against pathogens such as Group B Streptococcus infection by vaccinating their pregnant mothers, and the development of vaccines for potentially deadly adolescent and adult infections, including meningococcal disease, pneumococcal disease, and diseases caused by Staphylococcus aureus and Clostridium difficile.
Read more about how Pfizer is helping prevent infectious diseases to improve overall global health
Emerging Markets
PIH Emerging Markets focuses on the specialized needs of the developing and emerging world, using all of the resources available to us and our vast experience to bring Pfizer’s portfolio of innovative medicines and vaccines to patients and people in more than 100 markets across the world. Through unique commercial models and innovative solutions and partnerships, our goal is to accelerate access to the Pfizer innovative portfolio for patients and make a meaningful difference for the health and wellbeing of the more than five billion people living in emerging markets.
In 2017, the PIH Emerging Markets team obtained approval for and launched 40 innovative medicines and vaccines from its portfolio across our markets.
Explore our passion in action