
With One Infusion, a Path Toward Transforming Patient Lives With Gene Therapy Begins
Gene therapy offers the prospect of using one-time treatments to address an array of devastating diseases caused by genetic mutations. In July 2018, we initiated a late-stage clinical trial to help determine if one infusion of an investigational gene therapy will free hemophilia B patients from the regular injections they currently need to prevent spontaneous bleeding. And in March 2018, the first patient was dosed in a Phase 1b clinical trial for Pfizer's mini-dystrophin gene therapy candidate for Duchenne muscular dystrophy (DMD).
Although gene therapy holds tremendous promise, the science behind it is extraordinarily complex and presents many challenges. For example, using engineered viral vectors to introduce corrected copies of missing or defective genes into a patient's body requires expertise in translating the biology of a disease into meaningful therapeutic targets, expertise in engineering the vectors for the therapeutic genes, and expertise in manufacturing. We are addressing those challenges by advancing in-house resources and expertise, creating end-to-end capabilities from research and development to manufacturing and commercialization, and collaborating with leading biotech companies in the field. While the science is undoubtedly high-tech, the most powerful testimony to its potential may one day come from a 10-year old boy who can play a Little League season without once thinking about or being sidelined by his hemophilia.
"Pfizer is a leader in providing unprecedented and potentially transformational treatment for diseases that have been beyond the reach of medical intervention, and gene therapy is a prime example of that. We have advanced our in-house resources and expertise to explore the next generation of potential treatment options and the future of how we combat rare diseases."

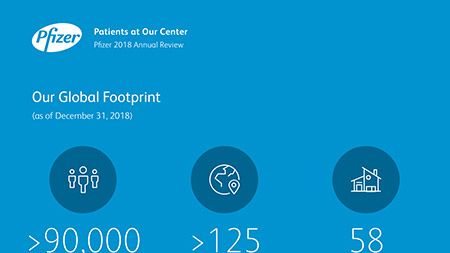
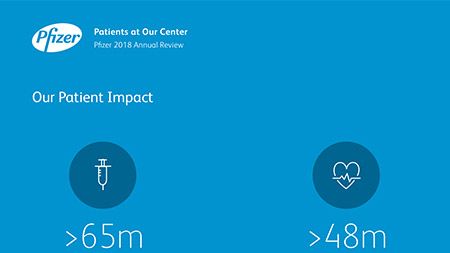
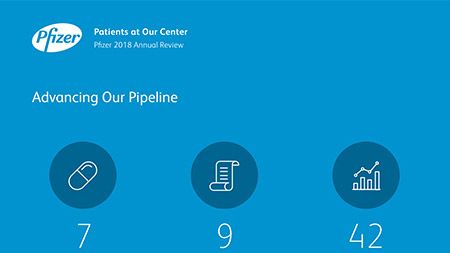



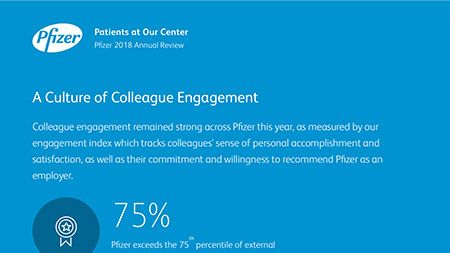




Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families