
Bringing Hope to Patients With a Rare Form of Heart Disease
The road to diagnosis can be a long and challenging one for patients with a rare disease, as is the case with transthyretin amyloid cardiomyopathy (ATTR-CM), a rare, fatal and underdiagnosed condition associated with progressive heart failure.
Pfizer has conducted the only Phase 3 study (ATTR-ACT) in ATTR-CM with our investigational treatment, tafamidis - a therapy which is also already approved under the name Vyndaqel™ in 40 countries and commercialized in 29 countries for the treatment of ATTR polyneuropathy. In 2018, we completed regulatory submissions to health authorities in Japan and the US for the use of tafamidis as a treatment option for patients with ATTR-CM, and additional submissions will continue to occur around the world in 2019. We support the Transthyretin Amyloidosis Outcomes Survey (THAOS) (www.thaos.net), the largest real-world international database for transthyretin amyloidosis, which collects data from people diagnosed with ATTR and carriers of ATTR mutation(s) in order to improve disease knowledge, understanding and management. We also work to further cardiologists' understanding of ATTR-CM and increase their knowledge of available diagnostics, including the development of a health care professional website in the U.S.: https://www.suspectanddetect.com/.

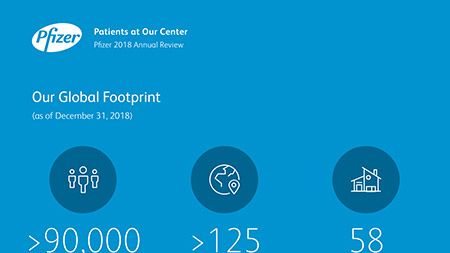
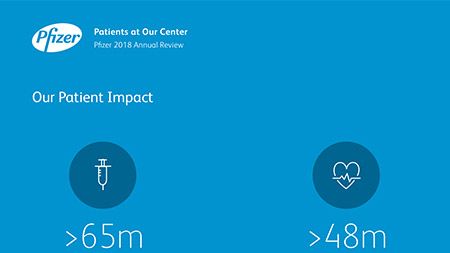
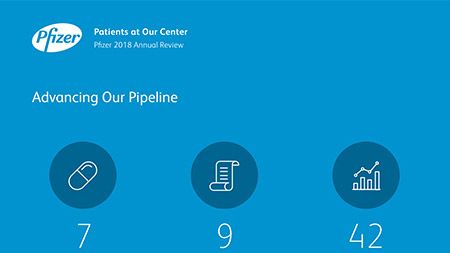



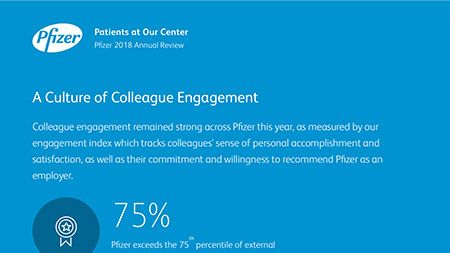




Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families