
Progressing Our Pipeline
As our understanding of the science and nature of disease evolves, Pfizer strives to address the needs of patients through our extensive portfolio of medicines and vaccines with a keen eye toward the future. Powered by innovative discovery research and development, our scientists take pride in inventing molecules that have the potential to meet patient needs and taking them through rigorous testing and, hopefully, registration and regulatory approval. We have built a broad pipeline of compounds that we believe hold the potential to deliver new, life-changing therapies for a wide range of debilitating and chronic diseases.
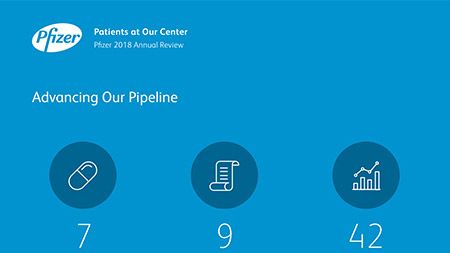
Additionally, our research and development teams dedicate their efforts to developing complex sterile injectables, new drug delivery systems, new formulations of existing therapies and expansion into new markets. Our global research pipeline - focused on areas where we believe we can make a significant contribution to patients – has 100 compounds in various stages of development. 37 percent of the 100 compounds are in Phase 3 trials or registration.
Discovery projects
Pfizer Pipeline as of January 2019
Spotlight on Immunology & Inflammation (I&I) Pipeline
Pfizer infuses the power of scientific innovation into our Inflammation & Immunology research and development efforts. We are working to transform the evolving treatment paradigm in inflammation beyond broad immunosuppression. In the clinic, we are targeting more selective inhibition of pro-inflammatory pathways to deliver potentially transformational outcomes for patients.
We have established leading kinase capabilities with multiple kinase inhibitor therapies in development. As a pioneer in Janus kinase (JAK) science, we're advancing several investigational programs with novel selectivity profiles, which, if successful, could potentially deliver transformative therapies for patients with rheumatoid arthritis, psoriasis, ulcerative colitis and alopecia areata.
Spotlight on Rare Disease Pipeline
The science that powers Pfizer's work in rare disease combines a deep understanding of disease biology, pioneering clinical research and ongoing dialogue with patients, who are steadfast partners in our work to translate science into medicine. Our goal is to develop effective therapies for rare diseases where there is the greatest treatment need and where we have unique, differentiated capabilities to bring potentially transformative options to patients. This includes the areas of hemophilia, sickle cell disease, amyloidosis, neuromuscular and inherited metabolic disorders.
We currently have more than 20 compounds in various stages of development in our rare disease pipeline (spanning 12 potential indications), and most have received Orphan Drug designation in the U.S. and EU. We have late-stage pipeline opportunities in transthyretin amyloidosis and sickle cell disease, and we are investigating highly specialized, potential one-time gene therapy treatments for diseases that have single gene defects, such as certain neuromuscular and hematologic diseases.
Spotlight on Vaccines Pipeline
Progressing Our Pipeline to Take on the Global Public Health Burden of Infections
Clostridium difficile. Clostridium difficile (C. difficile), is the most common cause of antibiotic-associated diarrhea in the health care setting and an increasing concern worldwide. The bacteria are present in the environment and can colonize the human intestine. When conditions are right, such as when patients have taken antibiotics, they can multiply and express toxins that lead to diarrhea, and in some cases, severe colon inflammation, tears in the gastrointestinal tract and death. The U.S. Centers for Disease Control (CDC) classified C. difficile as an urgent public health threat in 2013, for its association with antibiotic use and resistance.
Group B Streptococcus. Pfizer's Phase 1/2 trial of PF-06760805, our conjugate vaccine candidate to help protect against Group B Streptococcus (GBS) infection, which can manifest as a serious neonatal blood infection (sepsis), pneumonia and meningitis in newborns, with potentially fatal outcomes or long-lasting neurological damage in those infected, is ongoing. Women who are colonized with the GBS bacteria may pass it on to their newborns during labor and birth. This is the first clinical step in our efforts to explore the potential of immunizing women during pregnancy, a process known as maternal immunization, to protect their new babies from devastating infections.
Respiratory Syncytial Virus (RSV) Vaccine. In May 2018, Pfizer announced the start of a Phase 1/2 trial of our RSV vaccine candidate in healthy adult volunteers. RSV is a common respiratory virus that affects the lungs and airways, with significant impact on young children and older adults. The highest risk of severe outcome from RSV occurs in the first months of life. RSV affects 33 million children globally and leads to approximately 120,000 childhood deaths every year. In the U.S., approximately 177,000 older adults are hospitalized annually because of RSV. Our clinical program aims to develop a vaccine for populations at highest risk of infection: infants through maternal immunization, and older adults through direct vaccination.








Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families