How a Coronavirus Protease Inhibitor Works to Fight COVID-19
SARS-CoV-2, the virus that causes COVID-19, was sometimes called the “novel coronavirus” in the early days of the pandemic.
The term was fitting because SARS-CoV-2 was new; the world had not previously encountered this virus, though the scientific community was already quite familiar with other coronaviruses, including SARS-CoV-1, the virus that caused the 2003-2004 severe acute respiratory syndrome (SARS) outbreak, MERS-CoV that caused the Middle East respiratory syndrome (MERS) outbreak in 2012, as well as the common human coronaviruses responsible for billions of cases of the common cold.
There’s a reason we don’t yet have a cure for the common cold: viral infections are notoriously difficult to treat. That’s because viruses hide inside the cells of their hosts. Viruses hijack host cells and use the cells’ structures to crank out more copies of the virus, which then infect and hijack more hosts.
Destroying an invader that’s tucked into the cells of the body is a delicate task; you want to disable the invader without damaging the cell. Understanding the life cycle of viruses helps scientists develop medications that target the virus without affecting the host cells.
Breaking the SARS-CoV-2 life cycle
To summarize, for a virus to cause sickness in a human being, it must enter the body, then attach to and enter a host cell, then create new viral copies. Interrupting this process at any step may prevent infection, illness, or the spread of infection.
The virus that causes COVID-19, SARS-CoV-2, usually enters the body via the nose or mouth, but can also enter through the eyes by direct contact. The spike protein on the surface of the virus binds to cells that have a particular type of receptor on their surface. Like a key unlocking and opening a door, this connection allows the virus to enter the host cell.
Inside the cell, the virus releases its ribonucleic acid (RNA), a molecule that contains the genetic instructions that direct the cell to create proteins to make more viruses. The viral RNA is translated into a long protein chain called a polyprotein. Viral enzymes break the polyprotein into smaller protein units, which become the building blocks of new viral copies by replication of structural protein RNA. The new virus can then infect more cells.
The SARS-CoV-2 life cycle can potentially be stopped by interfering with any of these steps.
“You can inhibit the virus at the step of attachment,” says Devendra Rai, a senior scientist at Pfizer, “if you design a drug that binds to either the spike protein or the host receptor. Those are called entry inhibitors.”
Or “you can inhibit the virus at the step of making functional proteins by stopping the cleavage of the polyprotein,” Rai says. Medications aimed at stopping this step are protease inhibitors.
Another option is to stop the virus from making copies of its genetic blueprint. Medications that interrupt this step are called polymerase inhibitors.
The virus can also be inhibited at the assembly and release steps, which entail assembly of virus particle in the infected cell and its release from the infected cell, respectively. This is called viral release.
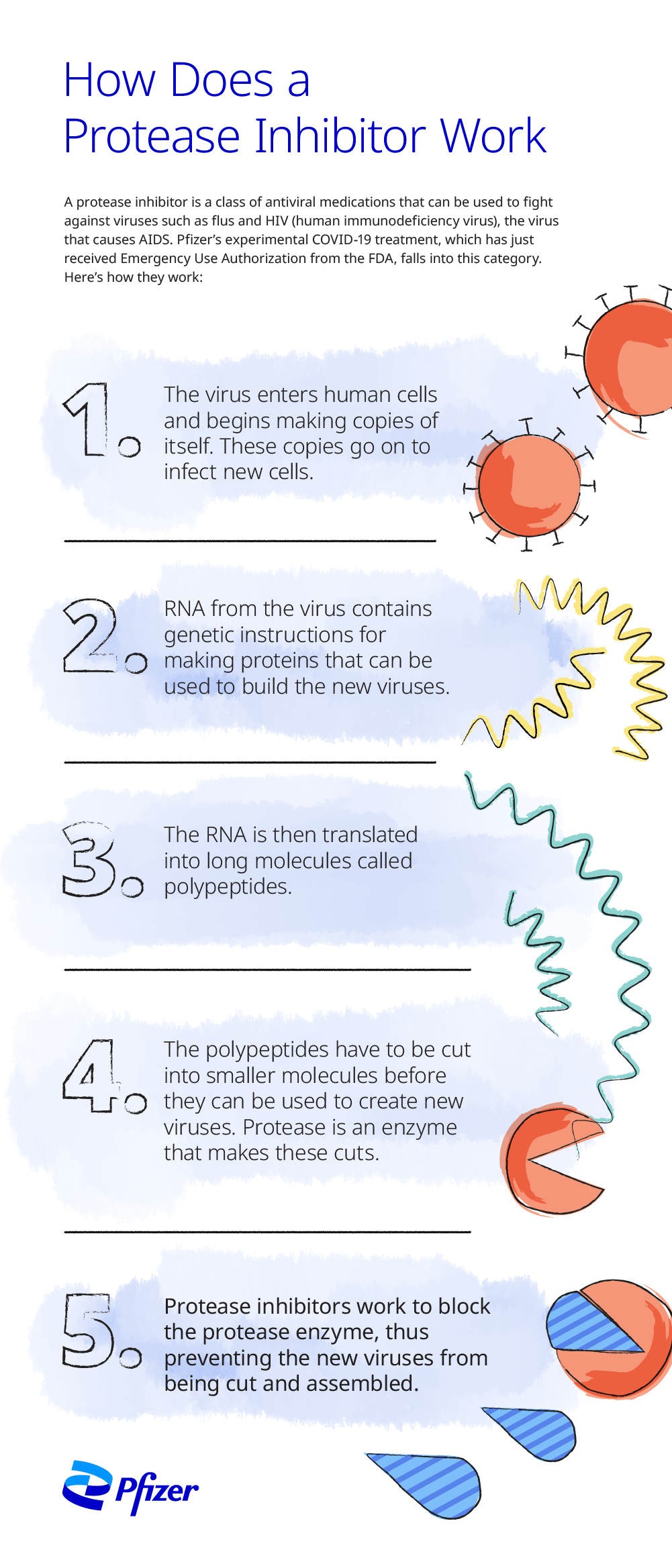
What is a protease inhibitor?
Medications that inhibit the cleavage of the polyprotein into functional proteins are called protease inhibitors. Protease is a protein-based enzyme that normally breaks the polyprotein into functional proteins, so blocking, or inhibiting, protease prevents this essential step of viral reproduction.
While the spike protein, the part of the virus that attaches to host cells, can be susceptible to mutations, the region of the polyprotein that produces protease is much less so, which means that it is far more likely to remain a stable target for a drug
“When you are designing an inhibitor, you want to design an inhibitor for a region that is not so readily mutated,” Rai says. The resulting protease inhibitor may thus retain efficacy versus variants of the same virus.
Pfizer's novel oral COVID-19 treatment is comprised of two protease inhibitors, nirmatrelvir and ritonavir that are administered together. Nirmatrelvir blocks SARS-CoV-2 main protease enzyme activity that would otherwise cleave the polyprotein into functional proteins.[1] Ritonavir, another protease inhibitor, is not active against SARS-CoV-2; it is used to slow the metabolism of nirmatrelvir, allowing it to be active for longer at higher concentrations so it can most effectively interrupt viral replication.[2]
The Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High Risk Patients) was a randomized, placebo-controlled trial in non-hospitalized adult subjects with a laboratory confirmed diagnosis of SARS-CoV-2 infection. It found that, when treated within three days of symptom onset, Pfizer’s oral treatment reduced the risk of COVID-19 related hospitalization or death from any cause by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19 .[3]
Consistent results were observed in the population treated within five days of symptom onset, with an 88% reduction of hospitalization or death. Treatment-emergent adverse events were comparable between the oral treatment (23%) and the placebo (24%), most of which were mild in intensity. Fewer serious adverse events (1.6% vs. 6.6%) and discontinuation of study drug due to adverse events (2.1% vs. 4.2%) were observed in patients dosed with the oral treatment, compared to the placebo, respectively.
On December 22, 2021, the U.S. Food and Drug Administration authorized the oral treatment for emergency use for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg, or 88 lb) with positive results of direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.[4]
The oral treatment should be initiated as soon as possible after a COVID-19 diagnosis has been made and within five days of symptom onset. The medication should be taken twice a day (morning and evening) for five days.[5]
We believe this medication may be an important tool to help fight COVID-19," Rai says. "I'm so proud to have played a role in that."
Pfizer's oral treatment for COVID-19 has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death. The emergency use of Pfizer's oral treatment for COVID-19 is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner. See EUA Fact Sheet: www.COVID19oralRx.com
[1] Paxlovid (nirmatrelvir and ritonavir) for the Treatment of COVID-19. Clinical Trials Arena. Accessed April 18, 2022. https://www.clinicaltrialsarena.com/projects/paxlovid-nirmatrelvir-ritonavir-covid-19/
[2] Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. U.S. Food & Drug Administration. Accessed April 18, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19.
[3] Pfizer. Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study [press release]. November 5, 2021.Accessed April 19, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate
[4] Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. U.S. Food & Drug Administration. Accessed April 18, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
[5] Frequently Asked Questions. Accessed April 18, 2022. https://www.covid19oralrx-patient.com/faq
![]()
