
Increased Investment in U.S. Manufacturing Will Help Deliver Essential Therapies and Create Jobs
We are experiencing an exciting new era in drug discovery and development with scientific advances that could result in future breakthroughs. To make this promise a reality, our manufacturing capabilities must keep pace with science today, and plan for what's coming next.
In 2018, we took an important step forward with the announcement of a $465 million investment to build a cutting-edge sterile injectable facility in Kalamazoo, Michigan. The 400,000-square-foot multistory building, known as Modular Aseptic Processing (MAP), will increase our ability to produce and supply important injectable medicines for patients in the U.S. and abroad.
"Our MAP investment helps ensure we continue to meet the evolving regulatory demands of every country where we do business," said Kalamazoo Site Leader Ron Perry. "Patients trust us to deliver the highest quality medicine in every dose."
The facility will incorporate technically advanced aseptic manufacturing equipment, systems and design, including multiple, self-contained modular manufacturing lines, and will create an estimated 450 highly skilled new jobs.
Groundbreaking is planned for spring 2019. Construction is expected to be completed in 2021, and production is expected to begin in 2024.
"This investment will strengthen Pfizer's leadership in sterile manufacturing technology and help meet growing patient demand. It also will create hundreds of highly skilled jobs, fortifying Michigan's high-tech manufacturing environment."

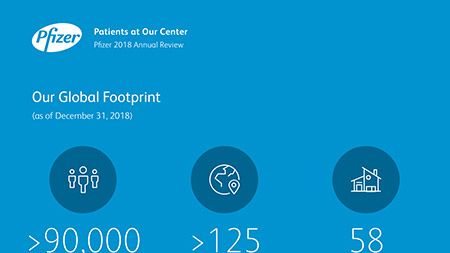
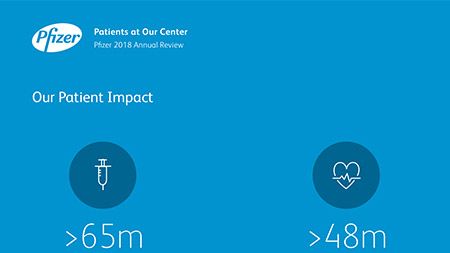
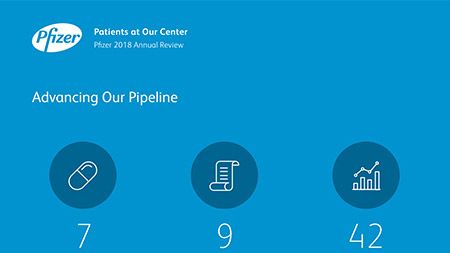



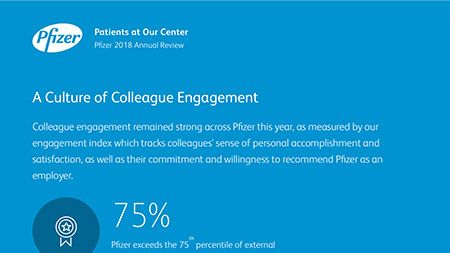





Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families