
Advancing Care Worldwide for People Living With Eczema
2018 marked the first global approval of Eucrisa® (crisaborole) ointment, 2 percent, the only 100 percent steroid-free topical phosphodiesterase-4 (PDE4) inhibitor that works above and below the skin's surface to treat eczema (a chronic and burdensome inflammatory disease). Eucrisa is now available for mild-to-moderate atopic dermatitis in patients 2 years of age and older in Canada, as well as in the U.S. Eucrisa offers patients a steroid-free prescription treatment option that can be used nearly anywhere on the body, as part of a long-term treatment plan. Additional regulatory submissions and approvals worldwide are anticipated in 2019 and beyond.

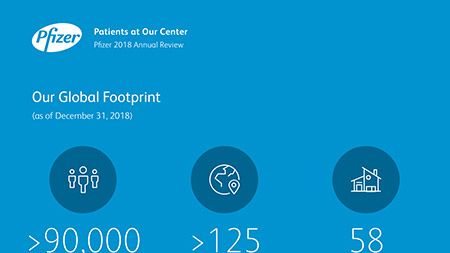
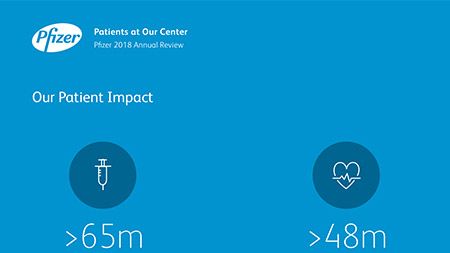
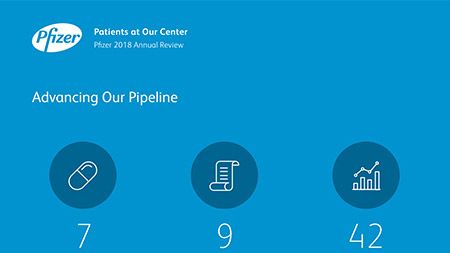



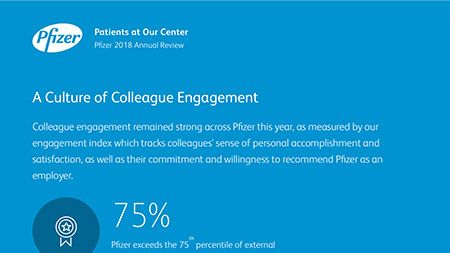




Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families