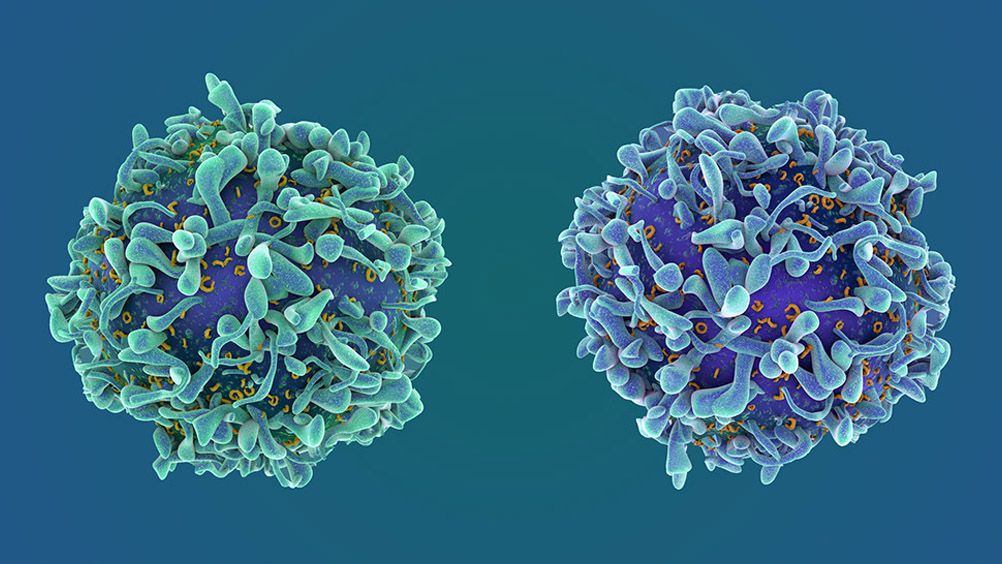
Biosimilars vs. Generics: What's the Difference?
A biologic medicine is derived from living organisms and is manufactured through highly involved and stringently controlled biotechnology processes. As biologic medicines, biosimilars are inherently different from generics due to their molecular size and structure, and the complexity and cost of their development. Biosimilars also have significantly higher research and development costs and risks and are more complex to manufacture than small-molecule generics.
Biosimilars have the potential to provide additional treatment options at lower cost, but development requires significant investment. Biosimilar development may take five to nine years and cost more than $100 million, not including regulatory fees. A generic, however, costs $1-2 million and takes approximately two years to develop.







Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
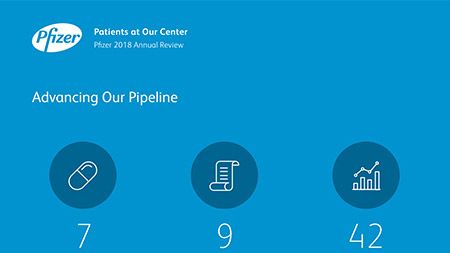
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families