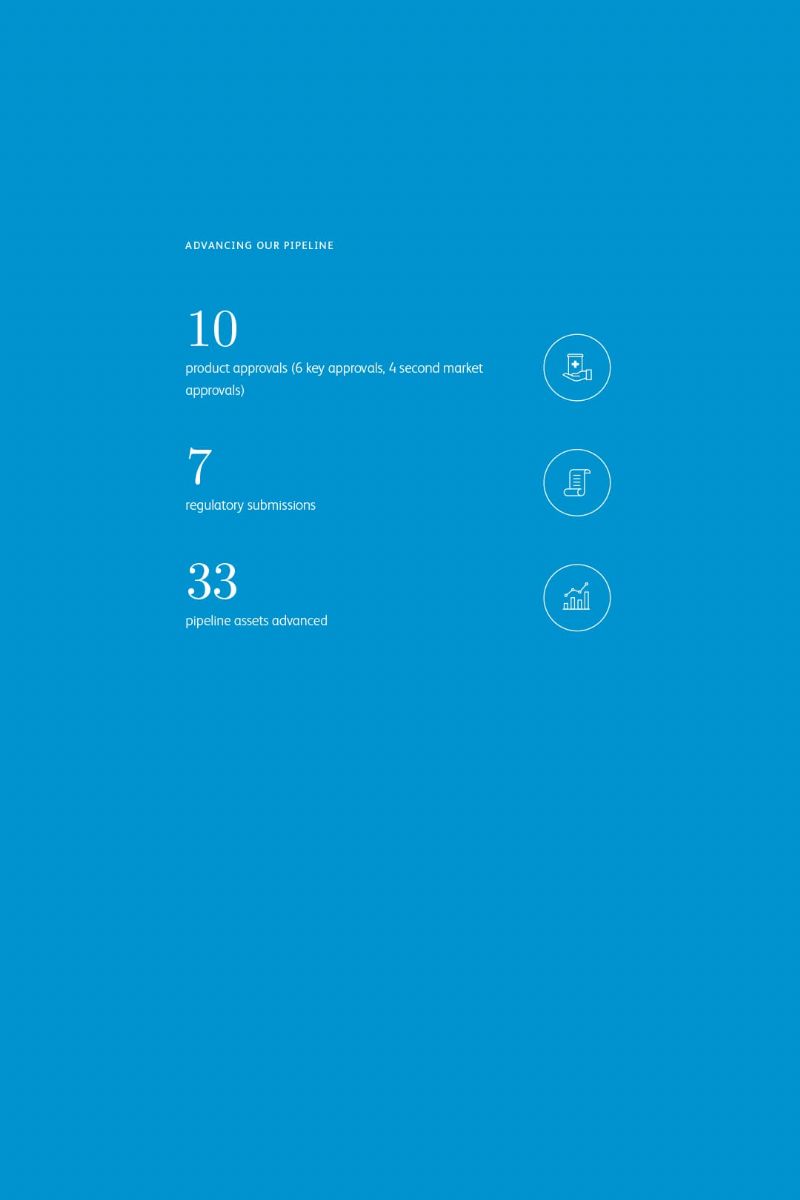
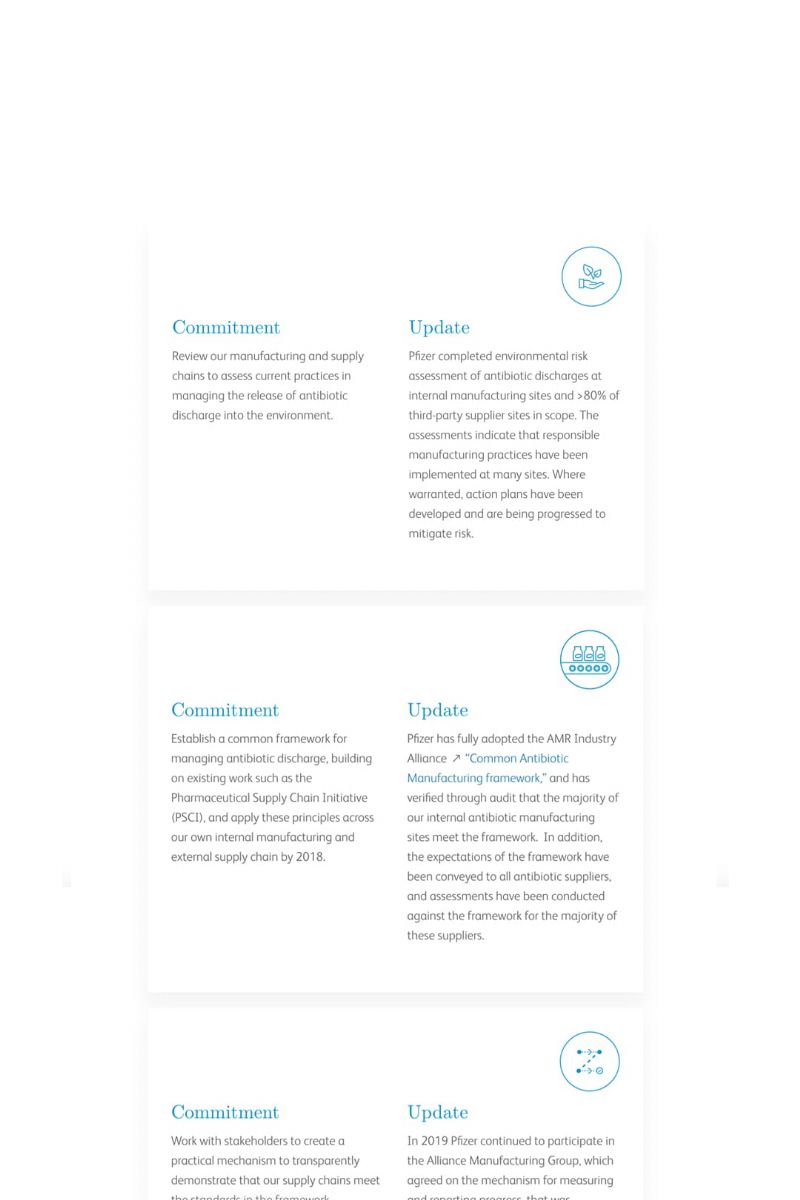
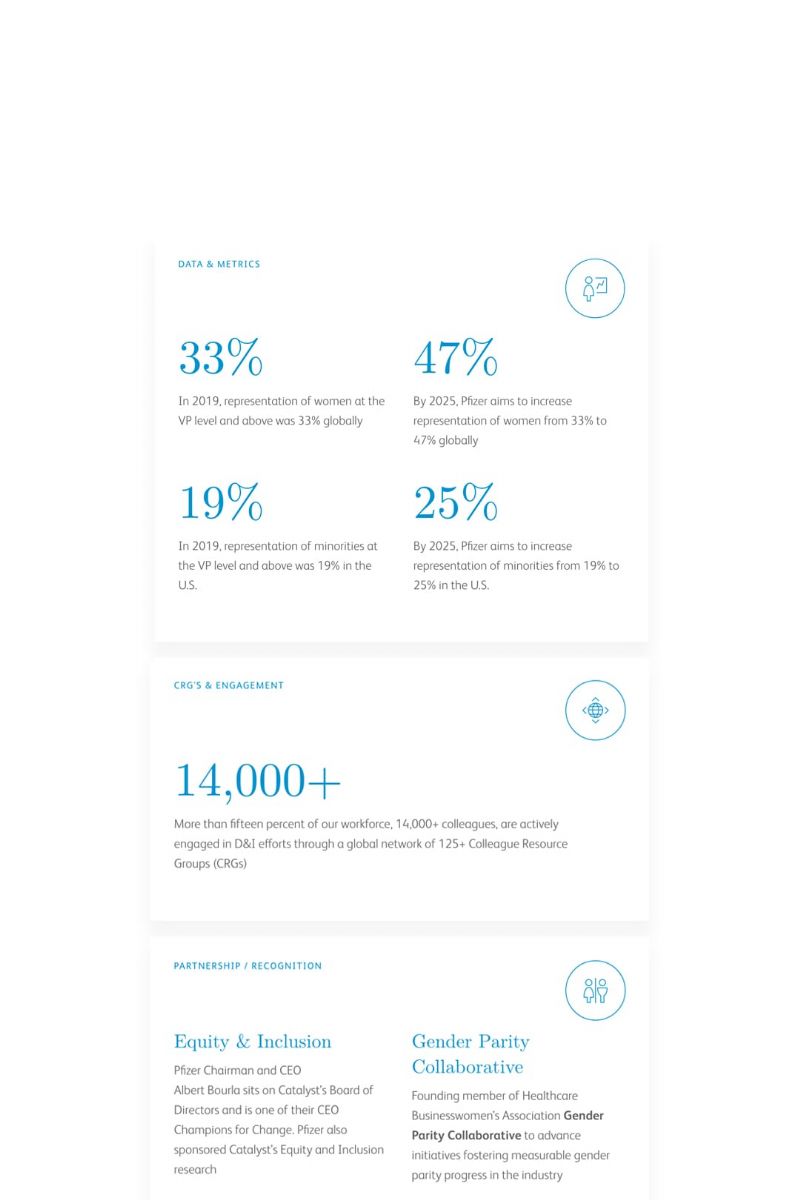
Some have called the 21st century “biology’s century.” For others, it’s “data’s century.” In Pfizer clinical development, it’s the best of both, as we apply digital advances and quantitative methods to improve the odds of success and accelerate drug development to deliver breakthrough medicines to patients faster.
Pfizer is using real-world data, alongside clinical data, for better insights into patient outcomes. We’re also applying biostatistical modeling to design trials that home in on the most relevant new data and to repurpose existing data for significant time and cost savings.
We’re automating repetitive processes, integrating clinical trial data systems and applying artificial intelligence to enhance our decision making.
We’re using digital tools to reach people who have not had access to trials before and including wearables and devices to help make trial participation easier for patients, as well as to collect more thorough and accurate data.
“By adopting new ways to recruit patients, automating processes, integrating systems, and using the totality of data to optimize our protocols, by 2021, we expect to reduce the average time it takes to develop a new medicine by about two years,” says Rod MacKenzie, Chief Development Officer, Pfizer.









.jpg)