
Using Breakthrough Science to Transform the Treatment of Cancer
At Pfizer Oncology, we believe the key to unlocking IO's full potential is by combining immunotherapies with targeted medicines based on unique tumor characteristics. In October 2018, Pfizer and Merck KGaA presented positive results from the Phase 3 JAVELIN Renal 101 clinical trial, among the first Phase 3 studies combining an immune checkpoint blocker with a tyrosine kinase inhibitor (TKI) in any tumor type. Results from the trial showed Bavencio® (avelumab) in combination with Inlyta® (axitinib) demonstrated significant improvements in progression free survival and objective response rates as compared with SUTENT® (sunitinib) in previously untreated patients with advanced renal cell carcinoma (RCC). These improvements were seen across a broad patient population with advanced RCC, including patients with favorable, intermediate or poor prognostic risk groups, and regardless of tumor PD-L1 expression. JAVELIN Renal 101 is continuing as planned to the final analysis for the other primary endpoint of overall survival (OS). No new safety signals were observed, and adverse events for Bavencio, Inlyta and Sutent in this trial were consistent with the known safety profiles for all three medicines.

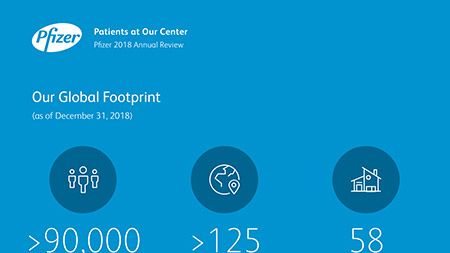
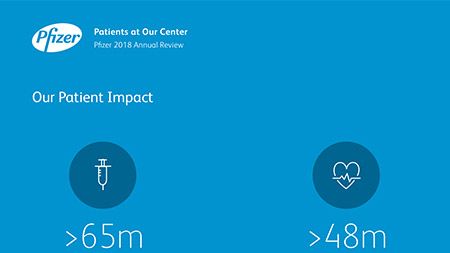
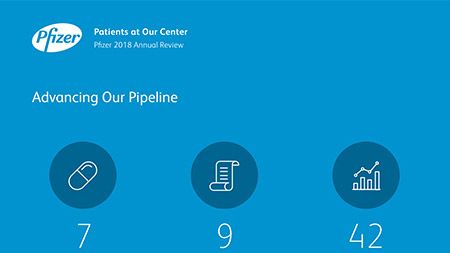



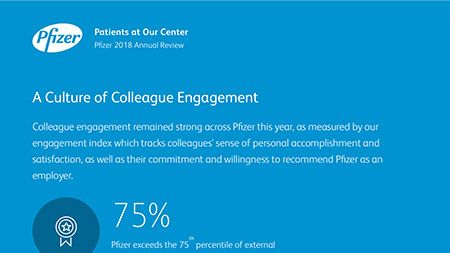




Ian Read: Contributions to Improving Human Health
Albert Bourla: Breakthroughs that Change Patients' Lives
Ushering in a New Era of Pfizer R&D Productivity
Advancing Our Leading JAK Science
Overcoming Therapy-Resistant Disease
Tackling Respiratory Syncytial Virus (RSV) Through Breakthrough Science and Technology
Catalyzing Innovations in Global Health
Contributing to the UN Sustainable Development Goals
Supporting Digital Health Start-Ups
Improving the Health of Women and Their Families