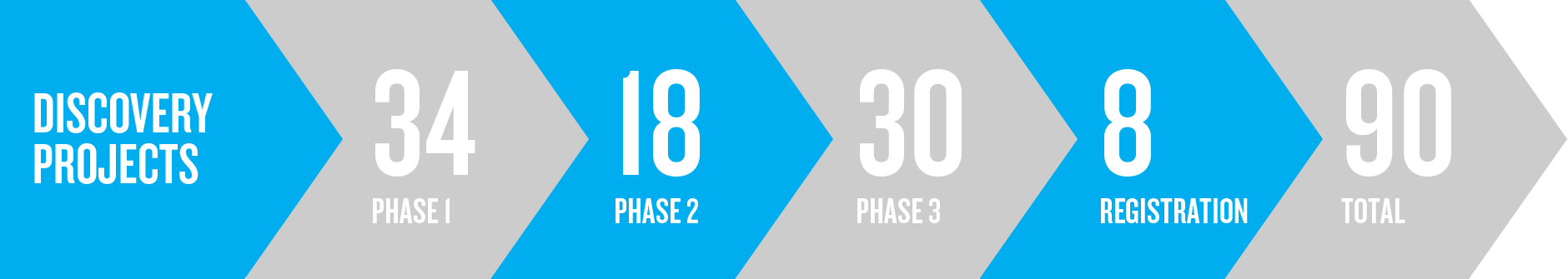
Our pipeline from Phase 1 to registration includes 90 investigational therapies, which are focused in areas where we have the potential to bring differentiated, high value therapies and vaccines to patients faster.
Our clinical research activities are focused on translating novel science into therapies and vaccines. Today, our clinical pipeline includes targeted immunotherapies, which have the potential to be part of the next generation of cancer therapy; first-in-class vaccines with the potential to help prevent two deadly hospital-acquired infections; antibodies that may be potentially useful in treating lupus and inflammatory bowel disease; and, a potential new therapy for Parkinson's disease. We are also applying our expertise in developing safe and effective biologic medicines to develop high quality biosimilars that may provide patients with access to alternative biologic therapies.