Hospira, Inc. Issues A Voluntary Nationwide Recall For 4.2% Sodium Bicarbonate Injection, USP ABBOJECT® Syringe; 8.4% Sodium Bicarbonate Injection, USP Lifeshield® ABBOJECT® Syringe; and Atropine Sulfate Injection, USP Lifeshield® ABBOJECT® Syringe
Recall due to the Potential For Presence of Glass Particulate Matter
FOR IMMEDIATE RELEASE - December 21, 2023 - NEW YORK, NY.
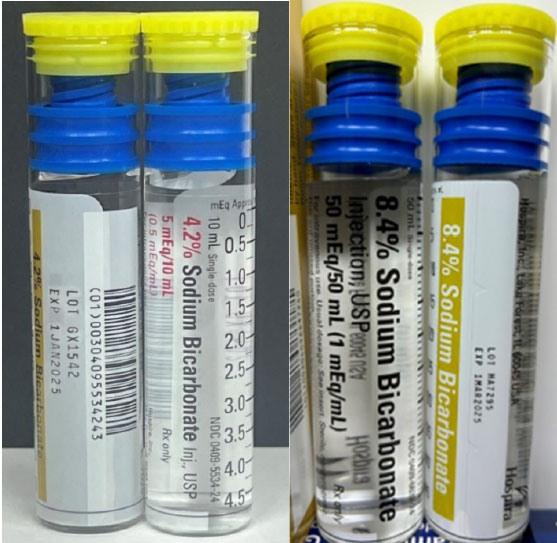
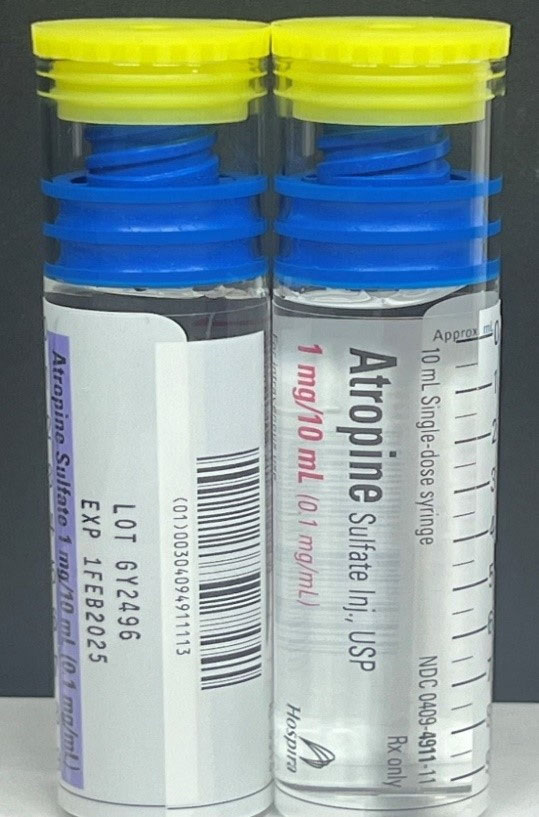
Hospira, Inc., a Pfizer company, is voluntarily recalling the lots listed in the table below of 4.2% Sodium Bicarbonate Injection, USP ABBOJECT® Glass Syringe, 5 mEq/10 mL; 8.4% Sodium Bicarbonate Injection, USP Lifeshield® ABBOJECT® Glass Syringe, 50 mEq/50 mL; and Atropine Sulfate Injection, USP Lifeshield® ABBOJECT® Glass Syringe, 1 mg/10 mL to the user level. The recall was initiated due to the potential for presence of glass particulate matter, identified during product inspection.
Should a patient receive an injectable product containing glass particulate matter as a result of this issue, the patient may experience serious adverse events. Potential complications related to injection of visible and subvisible inert particles include inflammation of a vein, granuloma, and blockage of blood vessels or life-threatening blood clot events. The frequency and severity of these adverse events could vary depending upon a variety of factors including the size and number of particles in the drug product, patient comorbidities (such as age, compromised organ function), and presence or absence of vascular anomalies.
The risk is reduced by the possibility of detection, as the label contains a clear statement directing the healthcare professional to visually inspect the product for particulate matter and discoloration prior to administration.
To date, Hospira, Inc. has not received reports of any adverse events associated with this issue for these lots. Sodium Bicarbonate Injection, USP is a sterile, nonpyrogenic, hypertonic solution of sodium bicarbonate (NaHCO3) in water for injection for administration by the intravenous route as an electrolyte replenisher and systemic alkalizer. It is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Sodium bicarbonate is further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturate-protein complex is desired), in poisoning by salicylates or methyl alcohol and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of hemoglobin and its breakdown products. It is also indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate.
Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate in water for injection with sodium chloride sufficient to render the solution isotonic. It is administered parenterally by intravenous injection and is indicated for temporary blockade of severe or life-threatening muscarinic effects, e.g., as an antisialagogue, an antivagal agent, an antidote for organophosphorus or muscarinic mushroom poisoning, and to treat bradyasystolic cardiac arrest.
The NDC, Lot Number, Expiration Date, and Configuration details for the impacted products are indicated below. The products were distributed nationwide to wholesalers/hospitals/institutions in the United States and Puerto Rico from March 14, 2023 through June 29, 2023.
| Product | NDC | Lot Number | Expiration Date | Presentation | Configuration / Count |
| 4.2% Sodium Bicarbonate Injection, USP ABBOJECT® Glass Syringe | Carton: 0409-5534-24 Case: 0409-5534-14 | GX1542 | 1JAN2025 | 5 mEq/10 mL (0.5 mEq/mL) | 1-10 mL Abboject Syringe per carton 10 cartons per bundle Case Pack 5 X 10-10 mL |
| 8.4% Sodium Bicarbonate Injection, USP Lifeshield® ABBOJECT® Glass Syringe | Carton: 0409-6637-24 Case: 0409-6637-14 | HA7295 | 1MAR2025 | 50 mEq/50 mL (1 mEq/mL) | 1-50 mL Abboject Syringe per carton 10 cartons per bundle Case Pack 5 X 10-50 mL |
| Atropine Sulfate Injection, USP LifeShield® ABBOJECT® Glass Syringe | Carton: 0409-4911-11 Case: 0409-4911-34 | GY2496 | 1FEB2025 | 1 mg/10 mL (0.1 mg/mL) | 1-10 mL Abboject Syringe per carton 10 cartons per bundle Case Pack 5 X 10-10 mL |
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Pfizer has notified direct consignees by letter to arrange for the return of any recalled product.
Wholesalers, hospitals, institutions, and doctors with an existing inventory of a lot which is being recalled should discontinue use, stop distribution and quarantine the product immediately. If you have further distributed the recalled product, please notify your accounts and/or any additional locations which may have received the recalled product. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Sedgwick Inc. at 1-800-805-3093 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the information below.
| Contact Center | Contact Information | Area of Support |
| Pfizer Medical Information | 1-800-438-1985, option 3 (9am to 5pm ET Monday through Friday) www.pfizermedinfo.com | For medical questions regarding the product |
| Pfizer Safety | 1-800-438-1985, option 1 (24 hours a day; 7 days a week) | To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form at www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form. Then complete the form and return it to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.
Media Contact:
Media Relations
+1-212-733-7410
[email protected]
# # # # #
4.2% and 8.4 Sodium Bicarbonate Injection, USP
Atropine Sulfate Injection, USP
05.01.2024 Albert Bourla Cancer Covid-19 Diversity & Inclusion Leadership Vaccines April Social Media Round-Up—Black Maternal Health, World Immunization, and Long Covid 04.30.2024 Cancer Covid-19 Leadership Technology Pfizer Goes Live in SXSW’s Podcast Lounge 04.09.2024 Albert Bourla Cancer Diversity & Inclusion Leadership March Social Media Round-Up—Annual Review, International Women’s Day, and a School of Science Celebration 04.05.2024 Breast Cancer Cancer Research Working to “Outdo” Cancer: A Multi-Modality Approach to Accelerating the Next Generation of Cancer Breakthroughs 03.27.2024 Health Equity Partnerships Rare Diseases Pfizer Teams With Black British Choir to Bring Awareness to Sickle Cell, Rare Diseases