Our Innovation
Anti-Infectives
Infectious diseases continue to be one of the largest threats to global public health. At Pfizer, we are driven by our desire to protect health and address the medical needs of people suffering from infectious diseases. Starting with our pioneering work on penicillin in the 1940s, we have a long and proud heritage of addressing evolving infectious disease challenges.
Infections can touch us all, at any age and in any country. We offer the industry’s largest and most diverse portfolio of anti-infectives, including more than 80 potentially life-saving medicines. We continue to invest in novel treatments for infectious diseases to address the greatest patient medical needs and, partnering with the infectious disease community, provide solutions that go beyond the medicine and offer innovative surveillance tools, shape the policy environment and support stewardship and education programs that help preserve the efficacy of the antimicrobials we already have available.
“As we look to 2018, we remain dedicated to expanding our longstanding leadership and expertise in the area of fighting infectious disease and, importantly, to continuing to address those areas of highest unmet medical need to better protect public health – now and in the future.”
Suneet Varma Pfizer Essential Health APAC, Greater China, Global Brands
Bringing Critical Anti-Infective Options to Patients and Physicians
Pfizer continues to invest in novel treatments for infectious diseases to address the greatest patient medical needs.
Most bacteria are classified as Gram-positive or Gram-negative. The latter are harder to treat due to their cell structure and their ability to develop resistance to commonly used antibiotics. In March 2017, we launched in the U.K. and Germany Zavicefta™ (ceftazidime-avibactam), a novel combination antibiotic for the treatment of patients with certain confirmed or suspected Gram-negative bacterial infections requiring hospitalization.
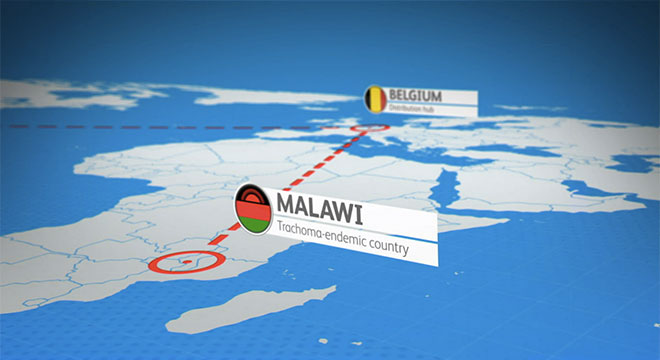
Zavicefta was acquired as part of the December 2016 deal with AstraZeneca PLC for its small molecule anti-infective business primarily outside the U.S. It was developed in response to the urgent medical need for new antibiotics for difficult-to-treat Gram-negative bacteria. Since March 2017, Zavicefta has launched in more than 14 countries. Additionally, we entered into an agreement with Basilea Pharmaceutica Ltd. for exclusive commercialization rights for Cresemba® (isavuconazole) in Europe, excluding Nordic countries, and for exclusive development and commercialization rights in China and several other countries in the Asia Pacific region. Cresemba is a novel anti-fungal treatment for adult patients with diagnosed invasive aspergillosis and mucormycosis, two serious infections caused by mold that are associated with high morbidity and mortality among immunocompromised patients.
As the global leader in anti-infectives, we are dedicated to the ongoing research and development of innovative products for patients with difficult-to-treat infections, including antibiotic-resistant infections. Pfizer is taking a leading role in public-private collaborations with the U.S. government and the EU Innovative Medicines Initiative to support the development of antibiotics to treat multi-drug resistant infections that pose an increasing global threat to public health. In addition, we are invested in expanding access to anti-infectives that help address areas of greatest unmet medical need, such as the treatment of Gram-negative infections and invasive fungal infections.
We expect to continue to make these important medicines available to many more patients across the globe throughout 2018.
Vaccines – A Partner in Addressing Antimicrobial Resistance (AMR)
In addition to the need for the development of new antibiotics and rational use of anti-infective medicines, experts agree that vaccines also play a vital role in addressing AMR.1 Vaccines are typically administered to help prevent infections from happening in the first place, which naturally leads to a reduction in the need for antibiotics. To date, several studies have demonstrated the beneficial role existing vaccines play in the reduction of AMR, and Pfizer is committed to continuing the development of new, innovative vaccines to help prevent serious disease globally.2
Commitment to Patients, Beyond Just Medicines
While anti-infectives have revolutionized medicine, the rapid spread of AMR is raising serious and acute concerns of life-threatening infections becoming impossible to treat and many routine medical procedures becoming too risky to perform. At Pfizer, we are deeply committed to leveraging our expertise and capabilities and working closely with the infectious disease community to address AMR through:
- Active stewardship to help ensure patients receive the correct antibiotic only if needed and for the right duration
- Global policy leadership to facilitate antibiotic development and proper use
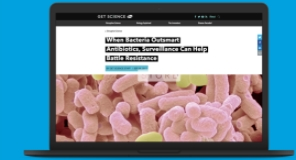
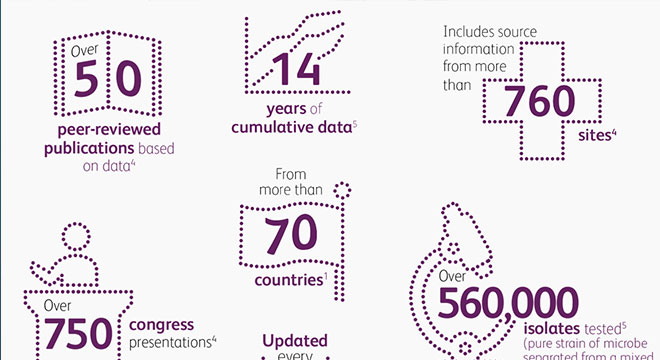
- Innovative surveillance tools (ATLAS) to help physicians better understand current resistance patterns, now offered as both an interactive, user-friendly website and as a mobile application enabling rapid access
- Working to expand a diverse portfolio of medicines and vaccines to treat and prevent serious infections around the world
- Responsible manufacturing practices that do not harm human health or the environment
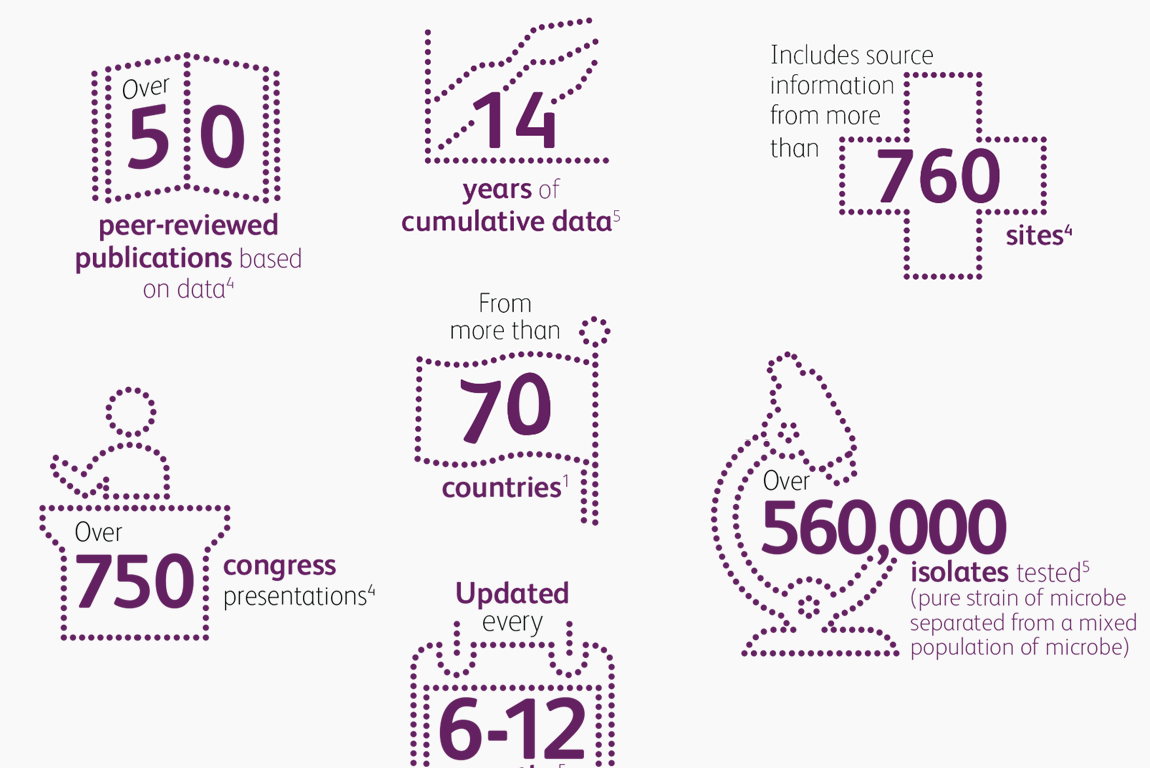
ATLAS Infographic
References
1. O’Neill, J. (2016). Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. Available from: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
2. Utt, Eric A. and Charles Wells (2016). The global response to the threat of antimicrobial resistance and the important role of vaccines. Pharmaceuticals Policy and Law 18 (2016) 179–197]
Explore our science in action