Our Innovation
Biosimilars
As one of the world's leading innovative biopharmaceutical companies, Pfizer is at the forefront of developing, manufacturing and delivering high-quality biosimilars to patients, physicians, and payers. Biosimilars have the potential to expand patient access to treatment and to create a more sustainable health care system. This should be achieved without compromising quality and by making resources available for further investment and innovation, so that more patients may receive the best possible care.
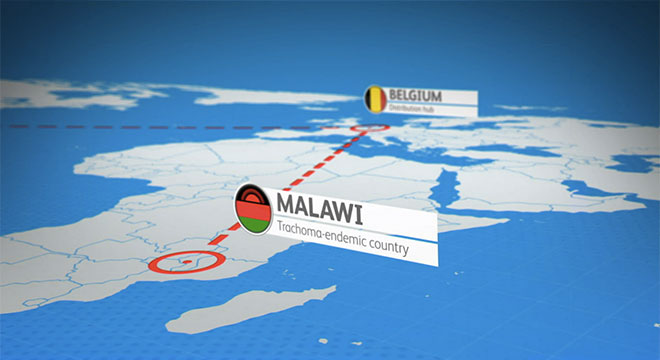
At Pfizer, we are making high-quality versions of existing biologic medicines that have lost exclusivity available to patients and physicians in a number of countries around the world across multiple life-threatening and chronic diseases in the fields of inflammation, immunology and oncology.
Our work in biosimilars reflects both our commitment to scientific innovation and our sense of urgency around patient access, and is a prime example of how we are driving value in every aspect of our business to improve patient health.
Pfizer has nearly 10 years of global experience developing biosimilars. Our current commercialized in-market portfolio includes Inflectra® (infliximab-dyyb in the U.S.), the first biosimilar monoclonal antibody to be approved in Europe and the U.S., and Nivestim® (filgrastim) and Retacrit® (epoetin zeta), both approved in Europe. In December, the U.S. Food and Drug Administration (FDA) approved Ixifi™ (infliximab-qbtx), also a biosimilar to Remicade® (infliximab).
Biosimilars vs. Generics: What’s the Difference?
A biologic medicine is derived from living organisms and is manufactured through highly involved and stringently controlled biotechnology processes. As biologic medicines, biosimilars are inherently different from generic medicines due to their molecular size and structure, and the complexity and cost of their development. Development of biosimilars is more difficult than that of small molecule generics, and generally requires more extensive clinical testing. Biosimilars also have significantly higher research and development costs and risks, and are more complex to manufacture than small-molecule generics. While biosimilars have the potential to provide additional treatment options at lower cost, development of biosimilars requires significant investment. Development of a biosimilar may take five to nine years at a cost of more than $100 million, not including regulatory fees. A generic version of a small-molecule drug, on the other hand, costs $1 million to $2 million and takes approximately two years to develop.
Building Upon a Heritage of Innovation
With more than 30 years of expertise in biologics, Pfizer has a deep understanding of the science behind these molecules and is a global leader in developing, manufacturing and delivering novel biologics and biosimilars that contribute to the treatment of millions of patients.
Building on our strengths in developing biologics, our biosimilars development pipeline is one of the largest such pipelines globally, with 12 total assets and seven assets in mid- to late-stage development noted in the chart below. Recent milestones include the acceptance for review of applications by the FDA and European Medicines Agency (EMA) for PF-05280014, our proposed biosimilar to Herceptin® (trastuzumab), and acceptance for review of our application by the FDA for PF-06881893, our proposed biosimilar to Neupogen® (filgrastim).
| Compound name | Mechanism of Action | Indication | Phase |
|---|---|---|---|
| Filgrastim, a potential biosimilar to Neupogen® (filgrastim) | Human Granulocyte Colony Stimulating Factor | Neutropenia in patients undergoing cancer chemotherapy (Biosimilar) | Registration |
| PF-05280014, a potential biosimilar to Herceptin® (trastuzumab) | erbB2 TK Inhibitor | Metastatic Breast Cancer (Biosimilar) | Registration |
| Retacrit®, a potential biosimilar to Epogen® and Procrit® (epoetin alfa) | Erythropoietin Stimulating Agent (ESA) | Treatment of Anemia (Biosimilar) | Registration |
| PF-05280586, a potential biosimilar to Rituxan® /MabThera® (rituximab) | CD20 Antigen Antagonist | Follicular Lymphoma (Biosimilar) | Phase 3 |
| PF-06410293, a potential biosimilar to Humira® (adalimumab) | Tumor Necrosis Factor Inhibitor | Rheumatoid Arthritis (Biosimilar) | Phase 3 |
| PF-06439535, a potential biosimilar to Avastin® (bevacizumab) | VEGF inhibitor | Non-Small Cell Lung Cancer (Biosimilar) | Phase 3 |
| PF-06881894, a potential biosimilar to Neulasta® (Pegfilgrastim) | Human Granulocyte Colony Stimulating Factor | Neutropenia in patients undergoing cancer chemotherapy (Biosimilar) | Phase 1 |
Our efforts to bring these important treatment options to market is driven by our belief that our science should always be focused on delivering high-quality medicines and doing what we can to help ensure that patients have uninterrupted access to the best possible care.
Explore our science in action