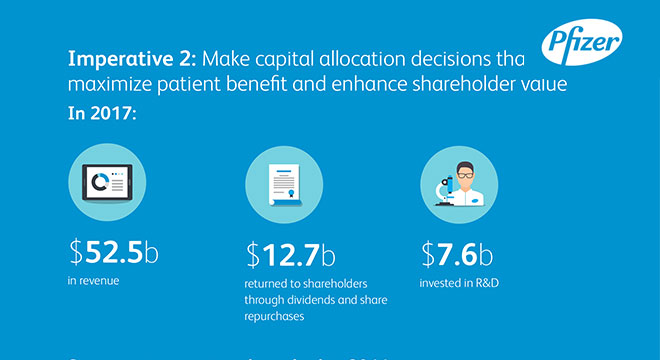
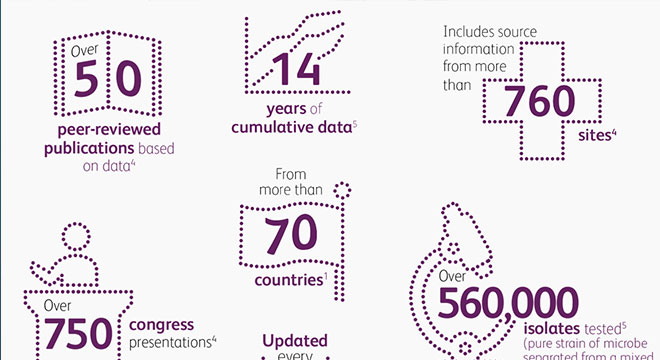
Our Innovation
Clinical Trials
Science and innovation are core to everything we do at Pfizer, and our drug development activities, including our clinical trials, are no exception. We readily embrace cutting-edge technologies, strategies and partnerships to help ensure our clinical trials are conducted with speed, agility and the highest attention to quality, to optimize our ability to bring innovative medicines and vaccines to patients as quickly as possible.
As technologies evolve, Pfizer is evolving drug development – the “D” in R&D. Development encompasses many activities from pharmacology to data management, but the heart of it is clinical trials – the rigorous process by which a proposed therapy is tested in humans, often over the course of many years, to assess safety and efficacy. As of the end of 2017, Pfizer had more than 300 active trials under way involving more than 55,000 patients.
Transforming Our Approach to Clinical Trials
Clinical trials have long been the most time-consuming, complex and expensive element of drug development. Today’s leaps in technology and ‘big data’ analytics, combined with breakthroughs in the understanding and medical application of human biology, are radically transforming the way drugs are developed; however, new methodologies must preserve the paramount goals of protecting patients and evaluating potential risks as well as benefits.
Pfizer is seeking to modernize clinical trials by pursuing what Rod MacKenzie, Pfizer’s Chief Development Officer, calls ‘extreme optimization.’ He explains: “We have one big job, and that is to serve our patients. To do that well, we have to work within the current clinical trials system and make it better, even as we work in parallel on more innovative ways to bring therapies forward.”
MacKenzie leads Pfizer’s Global Product Development (GPD) organization, created in 2016 to strengthen drug development at Pfizer as a discipline and make it a competitive advantage. MacKenzie and his leadership team set a strategy for becoming “best in class,” or among the very top performers in the industry in drug development. Since that time, in less than two years, Pfizer has already climbed into the top quartile on key drug development performance metrics.
Pfizer has also prioritized working directly with patients to plan how clinical trials may run. We are using new techniques and emerging digital technologies, from wearables and apps to ride-sharing services, to help accelerate and support patient recruitment – including among more diverse patient populations – and to make it easier and more convenient for patients to follow medication schedules and visit clinics.
Driving Diversity in Clinical Trials
This year, Pfizer launched an internal center of excellence within Pfizer to further establish diversity across our research portfolio. We took a multi-pronged approach to strengthen recruitment of under-represented patient populations in clinical trials, including a new investigator training program and revised recruitment and retention strategies, and engaged in key community-focused collaborations. Of particular interest are the Investigator Awareness Workshop, with videos to illustrate the importance of a diverse study population and barriers to minority enrollment and potential solutions, and the development of a Live Dashboard to inform the Pfizer development teams in real time about the diversity profile of the population in their ongoing clinical programs compared to the profile of the population with the disease being studied.
Explore our science in action