Our Innovation
Oncology
Pfizer’s advances in oncology exemplify the power of scientific innovation to transform lives. From immuno-oncology to cell therapy to the continued evolution of personalized medicine, our portfolio and pipeline of cutting-edge therapies aim to change the way the world approaches cancer treatment. We apply that same rigor to meeting the day-to-day emotional, financial and educational needs of these patients in order to help improve their lives.
In 2017, Pfizer Oncology achieved a large number of product approvals by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) and regulatory authorities around the globe. These approvals included:
- Bavencio® (avelumab) – in partnership with EMD Serono, the biopharmaceutical business of Merck KGaA – an immuno-oncology agent and the first immunotherapy approved for the treatment of metastatic Merkel cell carcinoma (mMCC), a rare and aggressive skin cancer, as well as for the treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy
- Besponsa® (inotuzumab ozogamicin) for the treatment of adults with relapsed or refractory β-cell precursor acute lymphoblastic leukemia
- Mylotarg® (gemtuzumab ozogamicin) for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia (AML) or for patients two years of age and older with CD33-positive AML who have experienced a relapse or who have not responded to initial treatment (refractory)
- Expanded indications for Ibrance® (palbociclib) in advanced or metastatic breast cancer
- Sutent® (sunitinib malate) in renal cell carcinoma
- Bosulif® (bosutinib) in newly-diagnosed chronic phase (CP) Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia
Mindful that patients need support on managing their life with cancer, we worked closely with the professional and advocacy communities to develop and expand programs such as Pfizer Oncology Together™, a one-stop shop for patients to get resources related to our therapies, and This Is Living With Cancer™, which provides additional tools for cancer patients, their families and caregivers.
Using Breakthrough Science to Transform the Treatment of Cancer
This year, Pfizer continued to accelerate its work in immuno-oncology (IO), which is an approach to cancer treatment that is designed to harness the natural ability of the body’s immune system to recognize and fight cancer.
As an emerging player in next-generation IO science, we recognize the urgency of our mission to help patients in need of anti-cancer alternatives. Pfizer is investigating a diverse array of compounds with the goal of helping more patients benefit from the power of IO.
In March, the FDA granted accelerated approval of Bavencio® as the first and only treatment for adults and pediatric patients 12 years of age and older with mMCC, a rare and aggressive skin cancer. Just six weeks later, the FDA also granted accelerated approval of Bavencio for the treatment of patients with locally-advanced or metastatic urothelial carcinoma (UC) who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. These indications are approved under accelerated approval based on tumor response rate and duration of response. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials. Bavencio, a human anti-PD-L1 antibody, is being developed through a collaboration by Pfizer and Merck KGaA, Darmstadt, Germany.
We have extended the reach of Bavencio in treating patients with mMCC beyond our initial success in the U.S., with approvals in Switzerland, the EU and Japan. The clinical development program for avelumab, known as JAVELIN, involves at least 30 clinical programs and more than 7,000 patients evaluated across more than 15 different tumor types.
In addition, by building unique doublet and triplet combinations, pairing IOs with each other or with targeted or traditional therapies, we have the opportunity to address difficult-to-treat cancers that don’t respond to currently approved immunotherapies, and we are exploring these combinations in several planned or ongoing trials. We currently have eight IO compounds in the clinic and are evaluating them as both monotherapies and as combination treatments.
Expanding Treatment Options in Breast Cancer
 In March, the FDA approved Pfizer's supplemental New Drug Application (sNDA) for Ibrance®, our first-in-class cyclin dependent kinase 4/6 (CDK 4/6) inhibitor. This FDA action broadened the range of anti-hormonal therapies that may be administered in combination with Ibrance. Ibrance now is indicated in combination with an aromatase inhibitor, expanding on its earlier indication in combination with letrozole, as initial endocrine-based therapy in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced or metastatic breast cancer. Ibrance also is approved in combination with fulvestrant in women with HR+, HER2- advanced or metastatic breast cancer whose disease progressed following endocrine therapy.
In March, the FDA approved Pfizer's supplemental New Drug Application (sNDA) for Ibrance®, our first-in-class cyclin dependent kinase 4/6 (CDK 4/6) inhibitor. This FDA action broadened the range of anti-hormonal therapies that may be administered in combination with Ibrance. Ibrance now is indicated in combination with an aromatase inhibitor, expanding on its earlier indication in combination with letrozole, as initial endocrine-based therapy in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced or metastatic breast cancer. Ibrance also is approved in combination with fulvestrant in women with HR+, HER2- advanced or metastatic breast cancer whose disease progressed following endocrine therapy.
Advancing the Science of Leukemia Treatment
 In December, the FDA approved Pfizer's sNDA for Bosulif® to expand the indication to include adult patients with newly-diagnosed chronic-phase (CP) Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML). The sNDA was reviewed and approved under the FDA’s Priority Review and accelerated approval programs based on major molecular response (MMR; the primary endpoint) and cytogenetic response rates (CCyR). Continued approval for this indication may be contingent upon verification and confirmation of clinical benefit in an ongoing long-term follow up trial. The EMA has also validated for review a Type II Variation application for use of Bosulif in the same patient population and the application is under review.
In December, the FDA approved Pfizer's sNDA for Bosulif® to expand the indication to include adult patients with newly-diagnosed chronic-phase (CP) Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML). The sNDA was reviewed and approved under the FDA’s Priority Review and accelerated approval programs based on major molecular response (MMR; the primary endpoint) and cytogenetic response rates (CCyR). Continued approval for this indication may be contingent upon verification and confirmation of clinical benefit in an ongoing long-term follow up trial. The EMA has also validated for review a Type II Variation application for use of Bosulif in the same patient population and the application is under review.
Addressing Areas of Unmet Need in Prostate Cancer
 In September, Pfizer and our commercial/development partner, Astellas, announced top-line results from the Phase 3 PROSPER trial of Xtandi® (enzalutamide) in patients with non-metastatic (M0) Castration-Resistant Prostate Cancer (CRPC). The trial showed that Xtandi, combined with standard of care Androgen Deprivation Therapy (ADT) versus ADT alone demonstrated a statistically significant improvement in metastasis-free survival (MFS) in this non-metastatic patient population.
In September, Pfizer and our commercial/development partner, Astellas, announced top-line results from the Phase 3 PROSPER trial of Xtandi® (enzalutamide) in patients with non-metastatic (M0) Castration-Resistant Prostate Cancer (CRPC). The trial showed that Xtandi, combined with standard of care Androgen Deprivation Therapy (ADT) versus ADT alone demonstrated a statistically significant improvement in metastasis-free survival (MFS) in this non-metastatic patient population.
Building on Our Commitment to Kidney Cancer Patients
 In November, the FDA approved Sutent® for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma (RCC) following nephrectomy. Sutent, which was approved more than a decade ago to treat advanced RCC, is the first approved adjuvant treatment in RCC, providing an option for patients that were previously limited to a wait-and-see approach.
In November, the FDA approved Sutent® for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma (RCC) following nephrectomy. Sutent, which was approved more than a decade ago to treat advanced RCC, is the first approved adjuvant treatment in RCC, providing an option for patients that were previously limited to a wait-and-see approach.
Leading a Call-to-Action in Advanced Metastatic Breast Cancer
In collaboration with the European School of Oncology, Pfizer announced in March the Global mBC Vision 2025 Call-to-Action to address the most pressing, pertinent and actionable gaps that patients with metastatic breast cancer (mBC) around the world face, as identified by a multi-stakeholder task force. Based on findings from the Global Status of Advanced/Metastatic Breast Cancer 2005-2015 Decade Report, the call-to-action was designed to unite the mBC community by catalyzing change to improve and extend the lives of patients with mBC by the year 2025. This important work has been taken forward by the ABC Global Alliance to form the ABC Global Charter.
Read more about the ABC Global Charter
Our #MBCVision 2025 Call-to-Action w/ @ESOncology aims to elevate #metastaticBC as a major global health concern #BCAM pic.twitter.com/HL3CLLYbfI
— Pfizer Inc. (@pfizer) October 18, 2017
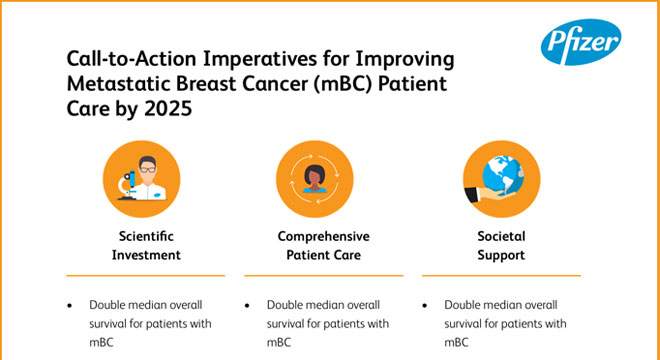
Imperatives for Improving Metastatic Breast Cancer Patient Care by 2025 Scientific Investment

 Scientific
Investment
Scientific
Investment
- Double median overall survival for patients with mBC
- Improve quality of life for patients with mBC in clinical practice
- Improve availability of good-quality epidemiology and outcomes data for mBC

 Comprehensive
Patient Care
Comprehensive
Patient Care
Comprehensive Patient Care
- Increase availability and access to multidisciplinary care, including palliative, supportive and psychosocial assistance, for patients, families and caregivers
- Strive for all patients with mBC to have financial support
- Offer communication skills training to all health care providers
- Provide mBC-specific information tools for every patient

 Societal
Support
Societal
Support
Societal Support
- Increase public understanding of mBC
- Improve access to non-clinical supportive services for mBC
- Protect workforce rights for patients with mBC
SPARC-ing Efforts for Change in mBC
In conjunction with Metastatic Breast Cancer Awareness Day on October 13, Pfizer and the Union for International Cancer Control (UICC) announced a new round of grants, totaling $500,000, to organizations taking part in the second phase of the Seeding Progress and Resources for the Cancer Community: Metastatic Breast Cancer Challenge (SPARC MBC Challenge). Of the more than 80 submissions received (from 42 countries), 20 organizations from 19 countries were selected to receive funding. The selected projects will be implemented to help close the gap in information, support, awareness and policy between mBC and early disease, as well as help reduce the number of women initially diagnosed at the metastatic stage of breast cancer. In addition, Pfizer is providing a further $30,000 to support the continuation of selected ongoing SPARC projects from the first round of grants issued in 2015.
Supporting the Needs of Cancer Patients in the Community
Today, more than 15 million people in the U.S. are living with cancer, and that number is expected to grow as additional therapies allow more patients to live with the disease. Building on our legacy of meaningful cancer support, Pfizer works closely with patient advocates, professional organizations and policy makers to better understand and meet the needs of these patients, their families and caregivers and others impacted by cancer.
We take a scientific approach to providing the educational, emotional and psychosocial resources that can help make a real difference in outcomes for cancer patients by leveraging insights and data to inform our content. We also provide millions of dollars in grants each year to fund organizations that provide essential programs and services to patients coping with a wide range of cancers.
Pfizer Oncology Together™
 To help patients better manage their lives with cancer, this year we launched Pfizer Oncology Together™, a first-of-its-kind program for patient access solutions and personalized support for those using Pfizer Oncology medicines in the U.S. As part of the program, patients will have access to a team of representatives with social work experience (“Care Champions”) who provide support beyond traditional access and reimbursement services by connecting them with a wide variety of patient education resources and outreach programs. The program’s services include identifying independent third-party resources that provide emotional support, lodging and transportation, providing tools to help with workplace transitions for patients balancing their jobs and treatment, and connecting patients and their families to local outreach programs. This service provides patients and caregivers with a dedicated representative over the course of their treatment.
To help patients better manage their lives with cancer, this year we launched Pfizer Oncology Together™, a first-of-its-kind program for patient access solutions and personalized support for those using Pfizer Oncology medicines in the U.S. As part of the program, patients will have access to a team of representatives with social work experience (“Care Champions”) who provide support beyond traditional access and reimbursement services by connecting them with a wide variety of patient education resources and outreach programs. The program’s services include identifying independent third-party resources that provide emotional support, lodging and transportation, providing tools to help with workplace transitions for patients balancing their jobs and treatment, and connecting patients and their families to local outreach programs. This service provides patients and caregivers with a dedicated representative over the course of their treatment.
This Is Living with Cancer™
 Continuing Pfizer’s efforts to put patients first by supporting those living with cancer and their caregivers, in October, we launched This Is Living with Cancer™, a program that provides tools and resources to support any patient who has been affected by cancer. The program includes a mobile app, LivingWith™, designed to help manage some of the daily challenges faced by people living with cancer. LivingWith provides patients and caregivers with a tool to organize certain important information in one place, including: build a network of support from friends and family to get help with daily tasks, record and remember important information from doctor visits, track mood/pain and connect with wearables, get organized and store key documents, receive information about local events and nutrition articles.
Continuing Pfizer’s efforts to put patients first by supporting those living with cancer and their caregivers, in October, we launched This Is Living with Cancer™, a program that provides tools and resources to support any patient who has been affected by cancer. The program includes a mobile app, LivingWith™, designed to help manage some of the daily challenges faced by people living with cancer. LivingWith provides patients and caregivers with a tool to organize certain important information in one place, including: build a network of support from friends and family to get help with daily tasks, record and remember important information from doctor visits, track mood/pain and connect with wearables, get organized and store key documents, receive information about local events and nutrition articles.
“Cancer patients can feel isolated and unsure about how to navigate care and approach day-to-day life. Pfizer’s new programming that goes beyond treatment is critical in helping patients and their care networks deal with the challenges associated with living with cancer.”
Myra Biblowit President and Chief Executive Officer, Breast Cancer Research Foundation
Breast Cancer: A Story Half Told®
Michael Kovarik, a retired elementary school teacher, radio host and author, became the first man to participate in the Story Half Told initiative to share his journey living with mBC and shine a light on the need for greater understanding of male breast cancer. Launched in 2014, the Story Half Told initiative aims to elevate public understanding of mBC, dispel misperceptions and combat stigma.
Facing Life: Voices of mBC Patients
In order to put a face to metastatic breast cancer, cancer care advocate and photographer Carolyn Taylor, founder of Global Focus on Cancer, traveled around the globe to photograph and interview more than 40 women with mBC in 12 countries. These interviews captured each woman’s unique story, perspective on life and the challenges she has faced while battling this disease. Learn more about their stories by visiting the patient gallery.
“Problems. Work. It doesn’t matter. We are the most important thing in our life, and sometimes we ignore that. It’s a crisis of conscience. Now, I devour life. I have to be happy every day. It changes everything.”
Karine Zamparo France
Cada Minuto Cuenta (Every Minute Counts)
mBC is the leading cause of death among women in Latin America. In an effort to raise awareness, support patients and address unmet needs, Pfizer partnered with 21 Latin American breast cancer advocacy organizations in an ongoing initiative called “Cada Minuto Cuenta (Every Minute Counts)." In Brazil, we distributed 2,000 patient materials across five major cities during Breast Cancer Awareness Month. Celebrities, cancer experts and athletes also supported this important initiative through videos shared on social media, and 637 media articles discussing mBC were published. These efforts reached 56 million Brazilians all over the country, showing that collaboration truly can improve patient lives, and make every minute count.
In Brazil:
Explore our science in action