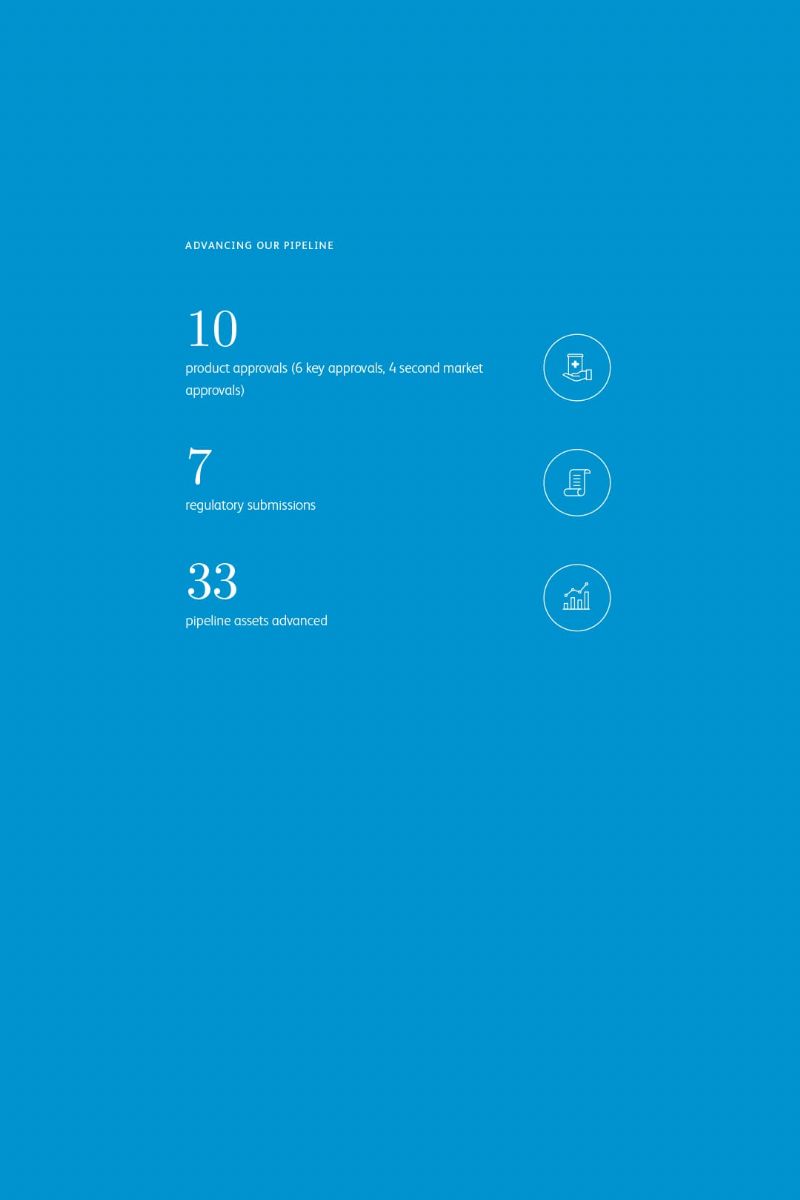
In April 2019,Ibrance® (palbociclib) was approved for men with HR+, HER2- metastatic breast cancer. While the U.S. Food and Drug Administration’s (FDA) decision was based largely on prior efficacy data from randomized controlled studies in women with metastatic breast cancer, such as the PALOMA-2 trial, Pfizer’s supplemental regulatory submission for male breast cancer primarily included real-world evidence.
Male breast cancer represents <1% of breast cancer cases, making trials and research surrounding treatment difficult. Pfizer engagedFlatiron Health to explore real-world data from post-marketing reports, Flatiron’s breast cancer database, electronic health records from IQVIA Insurance, Pfizer’s global safety database and controlled studies in women to make the case to the FDA that Ibrance could be used to treat men.
Related Articles
Unleash the power of our people
Deliver first-in-class science
Transform our go-to-market model
Win the digital race in pharma
Lead the conversation
Share this page
Why not also share...
Why not also share...
Goal 4
✕Quality Education
Ensure inclusive and equitable quality education and promote lifelong learning opportunities for all
Goal 12
✕Responsible Consumption & Production
Ensure sustainable consumption and production patterns
Goal 8
✕Decent Work and Economic Growth
Promote sustained, inclusive and sustainable economic growth, full and productive employment and decent work for all
Goal 16
✕Peace, Justice and Strong Institutions
Promote peaceful and inclusive societies for sustainable development, provide access to justice for all and build effective, accountable and inclusive institutions at all levels
Goal 9
✕Industry, Innovation and Infrastructure
Build resilient infrastructure, promote inclusive and sustainable industrialization and foster innovation
Goal 7
✕Affordable and Clean Energy
Ensure access to affordable, reliable, sustainable and modern energy for all
Goal 17
✕Partnerships for the Goals
Strengthen the means of implementation and revitalize the global partnership for sustainable development
Goal 6
✕Clean water and sanitation
Ensure availability and sustainable management of water and sanitation for all
Goal 2
✕Zero Hunger
End hunger, achieve food security and improved nutrition and promote sustainable agriculture
Goal 11
✕Sustainable cities and communities
Make cities and human settlements inclusive, safe, resilient and sustainable
Goal 14
✕Life below water
Conserve and sustainably use the oceans, seas and marine resources for sustainable development
Goal 15
✕Life on land
Protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss













.jpg)