Pfizer’s Pharmaceuticals in the Environment (PiE) Program (learn morehere) is advancing according to plan; with focus on assessment and wastewater management across our manufacturing supply chain.
We continue to advance EcoPharmaco Stewardship initiatives through our collaborations. Our participation in the Interagency PiE Task Force and its EcoPharmaco Stewardship (EPS) Initiative has been effective in shaping the EU PiE Strategy, which was published in March 2019 and commits to risk reduction actions that enable continued access to medicines. The Intelligence-led Assessment of Pharmaceuticals in the Environment (iPiE), a research project collaboration between Pfizer, other pharmaceutical companies and the Innovative Medicines Institute (IMI), delivered its project output in 2019. Key outputs are a database of environmental toxicity information and fate and effects models.
We continue to support patients with access to information aboutunused medicine disposal. Pfizer participates in the Pharmaceutical Product Stewardship Work Group (PPSWG), an industry coalition managing collection schemes in the U.S.
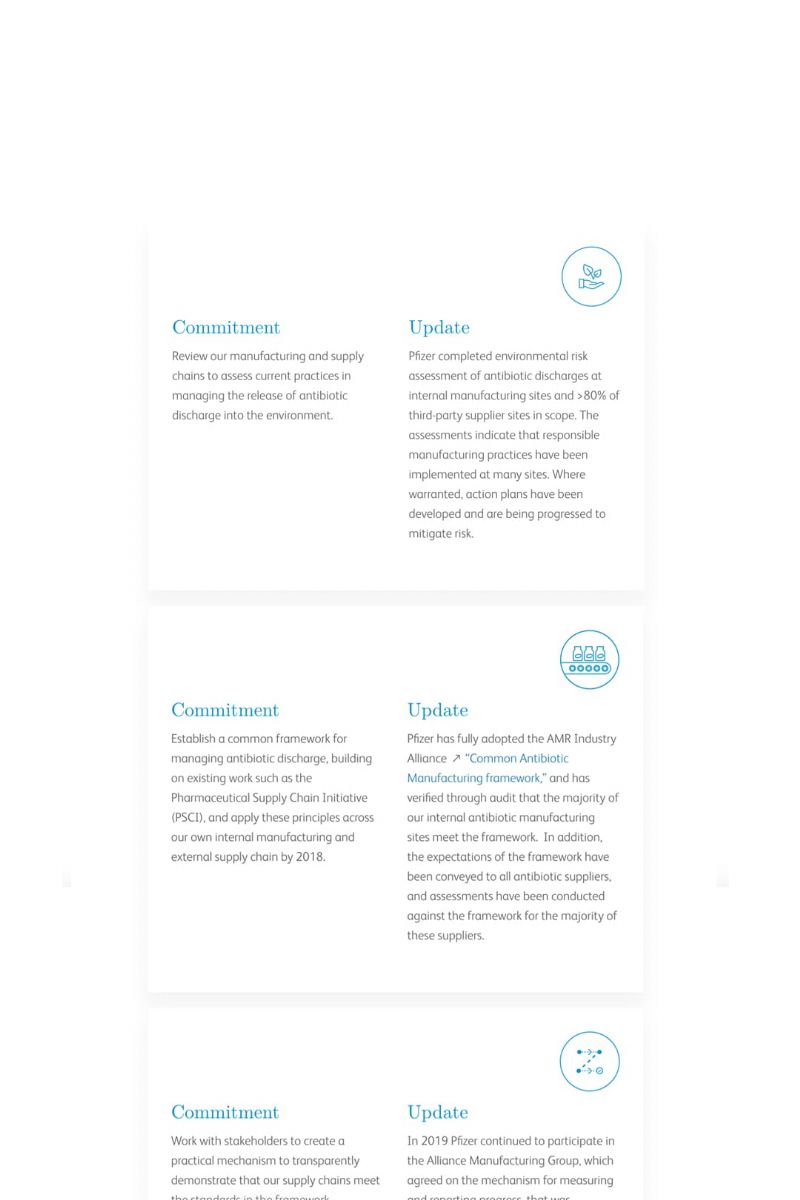
External collaboration remains a key component of our PiE program. Pfizer remains an active member of the Antimicrobial Resistance (AMR) Industry Alliance manufacturing group (the Alliance Manufacturing Group). Refer to the infographic below for progress made in 2019 with respect to AMR Industry Alliance commitments. Learn more about Pfizer’s policy position on AMR, including antibiotic manufacturing,here and learn more about the AMR Industry Alliance related to manufacturing and the environmenthere.
In 2019, we made the following progress against the AMR Roadmap commitments:
Review our manufacturing and supply chains to assess good practices in managing the release of antibiotic discharge into the environment.
Pfizer completed environmental risk assessment of antibiotic discharges at internal manufacturing sites and has nearly completed those assessments in the rest of the world. The assessments conducted indicate good practices are being followed at many sites. Where warranted, action plans have been developed and are being implemented to mitigate risk.
Establish a common framework for managing antibiotic discharge, building on existing work, such as the Pharmaceutical Supply Chain Initiative, and apply these principles across our own internal manufacturing and external supply chain by 2018.
Pfizer has fully adopted the AMR Industry Alliance“Common Antibiotic Manufacturing framework,” and has verified through an audit that the majority of our internal antibiotic manufacturing sites meet the framework. In addition, the expectations of the framework have been conveyed to all antibiotic suppliers, and assessments have been conducted against the framework for the majority of these suppliers.
Work with stakeholders to create a practical mechanism to transparently demonstrate that our supply chains meet the standards in the framework.
In 2019, Pfizer worked with the Alliance Manufacturing Group to agree on metrics/key performance indicators and gather performance data to include within an industry status report anticipated to publish in early 2020.
Work with independent technical experts to establish science-driven, risk-based targets for discharge concentrations for antibiotics and good practice methods to reduce the environmental impact of manufacturing discharges by 2020.
Pfizer continues to actively contribute as a founding member of the Alliance Manufacturing Group. In 2019, the Alliance established processes to evaluate new data and update, as necessary, the list of published risk-based discharge concentrations for a range of antibiotic compounds.