‘Doing Our Part’: A Colleague’s COVID Vaccine Trial Experience
The decision to join a clinical trial is an important and personal one. Thousands of people around the world volunteer for many reasons.
Bruce Altevogt, External Medical Engagement Lead for Pfizer Hospital, volunteered with his family for the COVID-19 clinical trial because he is as passionate about protecting them as he is about advancing scientific innovation.
“With COVID, we’re all looking for ways we could do our part, and this seemed like the obvious way in which we could contribute to the ongoing science and the ongoing work that Pfizer is committed to,” Altevogt said. “I trust our scientists, I know them personally, and I’m confident in what we’re doing and why we’re doing it.”

Sahana, Bruce, and Chaitan Altevogt at clinical trial appointment.
For his two children, Chaitan, 11, and Sahana, 9, participating in a clinical trial was an easy decision. They were excited to help others who had been affected by the COVID-19 pandemic and for the opportunity to leave the house for their clinical visits.
“It feels good helping other people,” said Sahana.
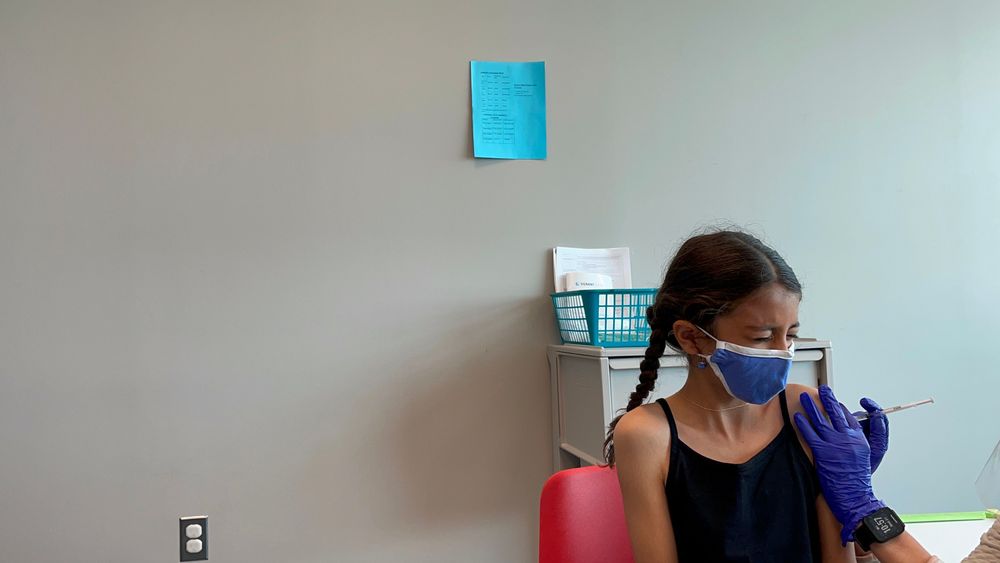
Sahana Altevogt gets her COVID-19 vaccine shot during her clinical trial appointment.
If you are considering joining a clinical trial, you’ll be connected with the research study team of medical professionals, including the study doctor and nurses. You may also have additional scheduled visits and procedures, extra laboratory tests, and/or follow a modified treatment plan.


