Charting a New Course for a COVID-19 Treatment
As COVID-19 continues to impact millions around the world, treatment options are still limited. At Pfizer, our scientists have focused on the discovery and development of a novel COVID-19 oral treatment that can now be prescribed at the first sign of infection or, subject to clinical success and authorization or approval, to avoid disease development following exposure to the virus.
Working with the utmost urgency to help lessen the impact of this devastating disease, we initiated this antiviral program – which originated and advanced in our labs – at the start of the pandemic, building a dedicated, multidisciplinary drug discovery team and using state-of-the-art computational and structure-based drug design capabilities to identify the right candidate to progress into the clinic. Within 16 months, we advanced that candidate from initial discovery efforts to regulatory submission for Emergency Use Authorization, one of the fastest development timelines in Pfizer history and a remarkable timeline for a small molecule therapy.
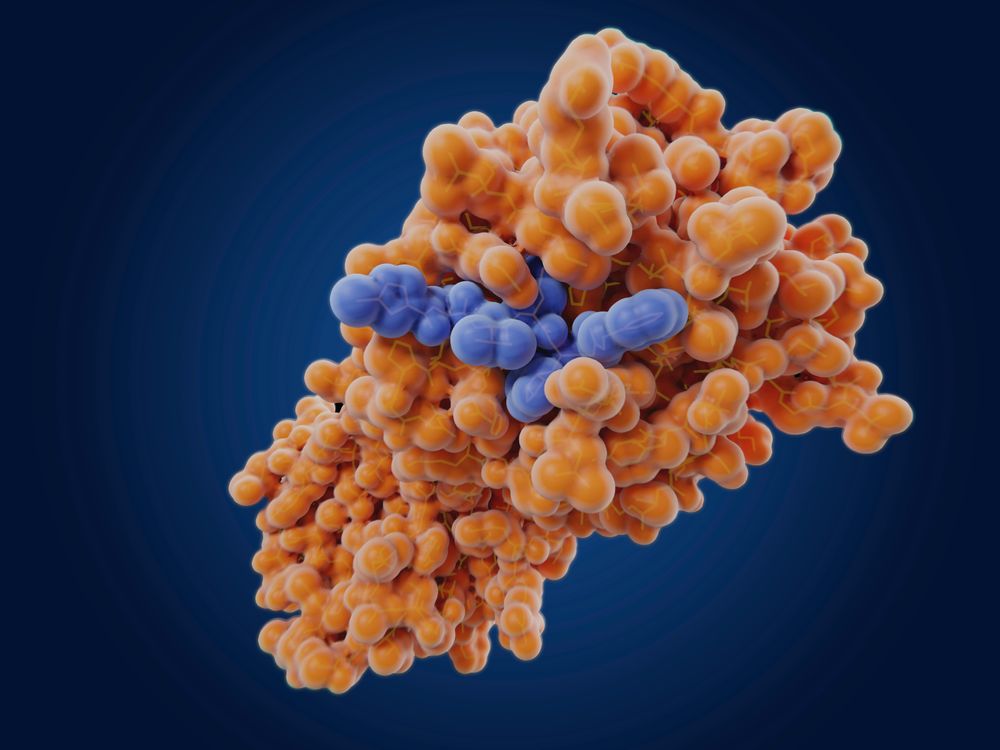
SARS-CoV-2 main protease and an inhibitor.
On December 22, the U.S. Food and Drug Administration (FDA) authorized the emergency use of Paxlovid™ (nirmatrelvir [PF-07321332] tablets and ritonavir tablets) for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg [88 lbs]) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization, or death.
This therapy is comprised of the first orally administered protease inhibitor specifically designed to target SARS-CoV-2, and several global Phase 2/3 trials commenced in the second half of 2021. Final results from the first of those trials, shared in December 2021, showed an 89 percent reduction in risk of hospitalization or death in high-risk patients, compared to placebo, within three days of symptom onset, with no deaths in the treatment group. In a secondary endpoint, the therapy reduced the risk by 88 percent, compared to placebo, with no deaths when treated within five days of symptom onset. Treatment-emergent adverse events were comparable between our oral therapy (23 percent) and placebo (24 percent), most of which were mild in intensity. These results were consistent with the interim analysis, which was announced in November 2021.
“Safe and effective treatment options represent the next big step in the fight against COVID-19 and will arm health care providers with the potential to save more lives.”
“Given the tremendous toll COVID-19 is taking on communities around the world, we knew we needed a development program with an ambitious global footprint, including nearly 7,000 participants from diverse backgrounds in North and South America, Europe, Africa, and Asia,” reflected James Rusnak, Senior Vice President and Chief Development Officer, Internal Medicine and Hospital. “Together with investigators at hundreds of sites, we’re working to move science forward, aiming to deliver a critical tool to help combat this pandemic.”
“Safe and effective treatment options represent the next big step in the fight against COVID-19 and will arm health care providers with the potential to save more lives,” said Annaliesa Anderson, Senior Vice President and Chief Scientific Officer, Bacterial Vaccines and Hospital. “We believe this novel, at-home therapeutic may help fill a critical treatment gap to reduce illness severity, hospitalizations, and death.”
Understanding the substantial need for both vaccines and treatment, we shouldered a significant financial risk to provide a breakthrough for patients that could help forge a new path to treating this disease.
“We have made a significant at-risk investment to support the manufacture and distribution of this new treatment for patients around the world,” shared Angela Lukin, Global President, Hospital, who also noted our commitment to working with governments and other groups, such as the Medicines Patent Pool, to help ensure access to all those who need treatment. “We’re working hard to get this treatment into the hands of patients as quickly as possible.”
Paxlovid™ has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg (88 lbs)) with positive results of direct SARS CoV-2 viral testing, and who are at high-risk for progression to severe COVID-19, including hospitalization or death.
The emergency use of Paxlovid™ is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner.
Pfizer Inc. Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment. December 22, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-receives-us-fda-emergency-use-authorization-novel. Accessed January 10, 2022.
Pfizer Inc. Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate. November 16, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-seeks-emergency-use-authorization-novel-covid-19. Accessed December 15, 2021.
Pfizer Inc. Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death. December 14, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Accessed December 15, 2021.
Pfizer Inc. Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study. November 5, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. Accessed December 15, 2021.
Pfizer Inc. Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries. November 16, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-medicines-patent-pool-mpp-sign-licensing. Accessed December 15, 2021.


