Hospira Inc. Issues A Voluntary Nationwide Recall For Buprenorphine Hydrochloride Injection Carpuject™ Units and Labetalol Hydrochloride Injection, USP Carpuject™ Units Due To The Potential For Incomplete Crimp Seals
FOR IMMEDIATE RELEASE – May 21, 2024 - NEW YORK, NY.,
Hospira, Inc., a Pfizer company (“Pfizer”), is voluntarily recalling the lots listed in the table below of Buprenorphine Hydrochloride Injection CarpujectTM Units and Labetalol Hydrochloride Injection, USP CarpujectTM Units to the User level. The recall was initiated due to the potential for incomplete crimp seals; one customer complaint has been received for one leaking unit.
In the event that impacted products are administered to a patient, there is a potential for an increased risk of lack of therapeutic effect and systemic infection that may result in the need for additional medical treatment. To date, Pfizer has not received reports of any relevant adverse events associated with this issue for these lots.
Buprenorphine HCl Injection is indicated for the management of pain requiring an opioid analgesic and for which alternate treatments are inadequate. Buprenorphine HCl Injection is a clear, sterile, injectable agonist-antagonist analgesic intended for intravenous (IV) or intramuscular (IM) administration.
Labetalol HCl Injection is indicated for control of blood pressure in severe hypertension.
The NDC, Lot Number, Expiration Date, and Configuration details for the impacted products are indicated below. The products were distributed nationwide to wholesalers/hospitals in the United States from September 2023 through April 2024.
| Product | NDC | Lot Number | Expiration Date | Concentration | Configuration/Count |
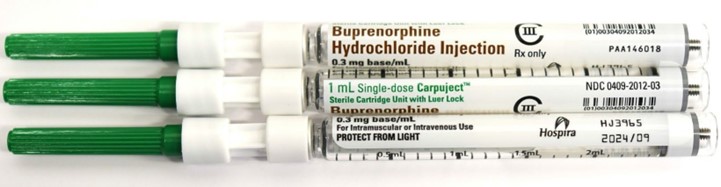
| Buprenorphine Hydrochloride Injection – CIII CarpujectTM Single-dose Cartridge/Tube Unit with Luer Lock | Carton 0409-2012-32 Cartridge 0409-2012-03 | HJ3965 | 2024/09 | 0.3 mg base/mL | 10 cartridge units/carton |
| HJ8546 | 2024/10 | 0.3 mg base/mL | 10 cartridge units/carton | ||
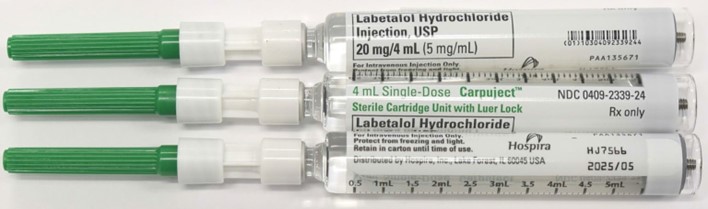
| Labetalol Hydrochloride Injection, USP CarpujectTM Single-dose Cartridge Unit with Luer Lock | Bundle 0409-2339-34 Carton/ Cartridge 0409-2339-24 | HJ7566 | 2025/05 | 20 mg/4 mL (5 mg/mL) | 10 carton/ cartridge units/bundle |
| HN8747 | 2025/09 | 20 mg/4 mL (5 mg/mL) | 10 carton/ cartridge units/bundle | ||
| HN8749 | 2025/09 | 20 mg/4 mL (5 mg/mL) | 10 carton/ cartridge units/bundle |
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Pfizer has notified direct consignees by letter to arrange for the return of any recalled product.
Wholesalers and hospitals with an existing inventory of any of the lots which are being recalled should discontinue use, stop distribution, and quarantine the product immediately. If you have further distributed the recalled product, please notify your accounts and/or any additional locations which may have received the recalled product. Hospitals should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Sedgwick at 800-805-3093 between the hours of 8a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the information below.
| Contact Center | Contact Information | Area of Support |
| Pfizer Medical Information | 800-438-1985, option 3 (Mon.-Fri. 9am – 5pm ET) www.pfizermedinfo.com | For medical questions regarding the product |
| Pfizer Drug Safety | 800-438-1985, option 1 (24 hours a day; 7 days a week) | To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form at www.fda.gov/MedWatch/getforms.htm or call 800-332-1088 to request a reporting form. Then complete the form and return it to the address on the pre-addressed form or submit by fax to 800-FDA-0178.
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.
# # # # #
Buprenorphine Hydrochloride Injection – CIII
1 mL CarpujectTM Single-dose Cartridge units with Luer Lock
Labetalol Hydrochloride Injection, USP
4 mL CarpujectTM Single-dose Cartridge units with Luer Lock
Media Contact:
Media Relations
+1-212-733-7410
[email protected]
02.19.2026
02.17.2026
02.05.2026
02.04.2026
02.02.2026
