Our Quality Policy
Our Corporate Quality Policy describes how we ensure the delivery of safe and effective drug, biologic, consumer, medical device and combination products and meet regulatory requirements and other relevant standards and guidelines. It provides a systematic approach designed to ensure we meet our commitment to patients and it defines the elements of our Quality Management System (QMS) for regulated activities.
Our commitment to developing, manufacturing, and delivering safe and effective products to patients includes:
- Maintaining an effective QMS within and across divisions
- Complying with all applicable quality regulations, codes and standards
- Maintaining a quality and integrity focused culture in which each person is required to carry out their responsibilities with proper regard for quality and to ensure the highest priority is placed on the safety and efficacy of our products, the safety of our patients, the integrity of data, and the trustworthiness of interactions with our Stakeholders
- Reporting and addressing quality issues, risks and concerns through appropriate corporate or divisional processes.
Our Commitment to Delivering Quality Products
We set high standards for our product quality and safety because behind each dose is a person—a parent, healthcare worker, friend, or child—who depends on us. Meet some of the individuals working behind the scenes, helping to ensure each and every dose meets our quality standards.
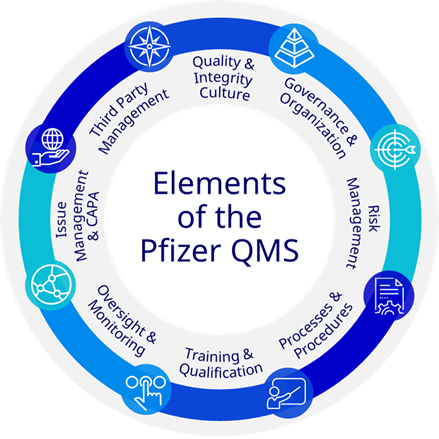
Elements of the Pfizer Quality Management System
- Quality & Integrity Culture
A robust culture of integrity and accountability is in place in which quality and compliance are seamlessly integrated into day-to-day business processes and activities to help ensure the highest priority is placed on the safety of our patients, quality of our products, integrity of our data, and Pfizer’s reputation.
- Governance & Organization
Senior leaders with the appropriate accountability and decision-making authority periodically review the performance of the QMS to ensure that it continues to fulfill quality objectives, remains aligned with the strategic direction of the organization, is supported by appropriate resources, and provides a pathway for escalation of quality, safety and compliance issues and risks.
- Risk Management
Known and emerging quality, safety and compliance risks are identified, assessed, escalated, and mitigated.
- Processes & Procedures
Processes, policies and procedures and any associated changes are managed in a controlled manner, commensurate with the level of risk.
- Training & Qualification
All individuals who perform work for, or on behalf of, Pfizer have the appropriate education, training, skills, qualifications, and experience to carry out their work competently, in accordance with all applicable laws, regulations, and Pfizer policies and procedures.
- Oversight & Monitoring
Programs and plans are in place for monitoring process performance, product quality, safety and compliance with applicable regulations and Pfizer policies to ensure a state of control is maintained.
- Issue Management & CAPA
Quality issues are investigated, with effective corrective and preventative actions (CAPA) implemented. Mechanisms are in place for timely escalation of critical quality, safety, and compliance issues.
- Third Party Management
Controls are in place to manage risks that originate from, or are related to, dependence on external entities, including third parties conducting work on Pfizer's behalf and other partnership arrangements, designed to ensure compliance with all applicable regulations and Pfizer policies.
How Drugs Are Made

Today's medicines have, on average, taken 12 years to go from inception to market. We believe that with dedication, creativity, and science, we can significantly reduce that time.





