Press Releases
All Pfizer published press releases by date and/or category of news
02.17.2026
02.06.2026
02.05.2026
Trust in Technology – A Snapshot
Pfizer surveyed adults in 10 countries across the Global North and South to better understand the general population's trust in incorporating technology into the management of their healthcare. The results showed that 6 in 10 trust technology in this setting but that means 4 in 10 are at risk of being left behind. Our findings underscored that patients won’t feel the full benefits of that technology if we don’t have trusted people – specifically health care providers – delivering it. We must partner with patients, healthcare providers, policy makers and others in the life sciences and tech industries to build trust in the technologies that are fueling the future of health.
Downloadable Infographic Details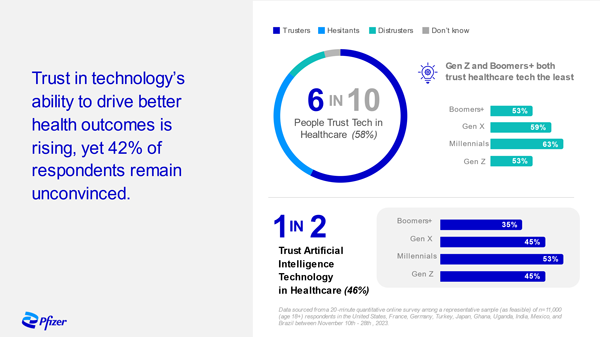
Media Asset Library
A collection of assets for use by media.
Partnering News
The latest news from Pfizer and its strategic partners
12.01.2025
10.22.2024
Media Contacts
Pfizer Media Relations
We encourage everyone to view our press releases, press statements, and press kits to stay up to date on Pfizer news.
However, to ensure that customers, investors, and others receive the appropriate attention, Pfizer media contacts may only respond to calls and emails from professional journalists.
Media Contacts
General email
[email protected]
United States, Canada and Latin America
+1 212.733.1226
Europe, the Middle East and Africa
+44-(0)173.733.2335
[email protected]
Asia Pacific
+65.918.732.47
What we need
To best help us help you, please be prepared to provide your name and email address, a summary of the article you're working on and your deadline.
Other Frequently Visited Links
For more, see these helpful and often used sections of Pfizer.com
Pfizer Wire
Stay up to date on the latest news and alerts through the Pfizer Wire
Sign up to receive real-time updates on Pfizer’s news from the Pfizer Media Relations team delivered directly to your inbox.
Sign Up Now Details


