Albert Bourla Reflects on the One Year Anniversary of the COVID-19 Pandemic
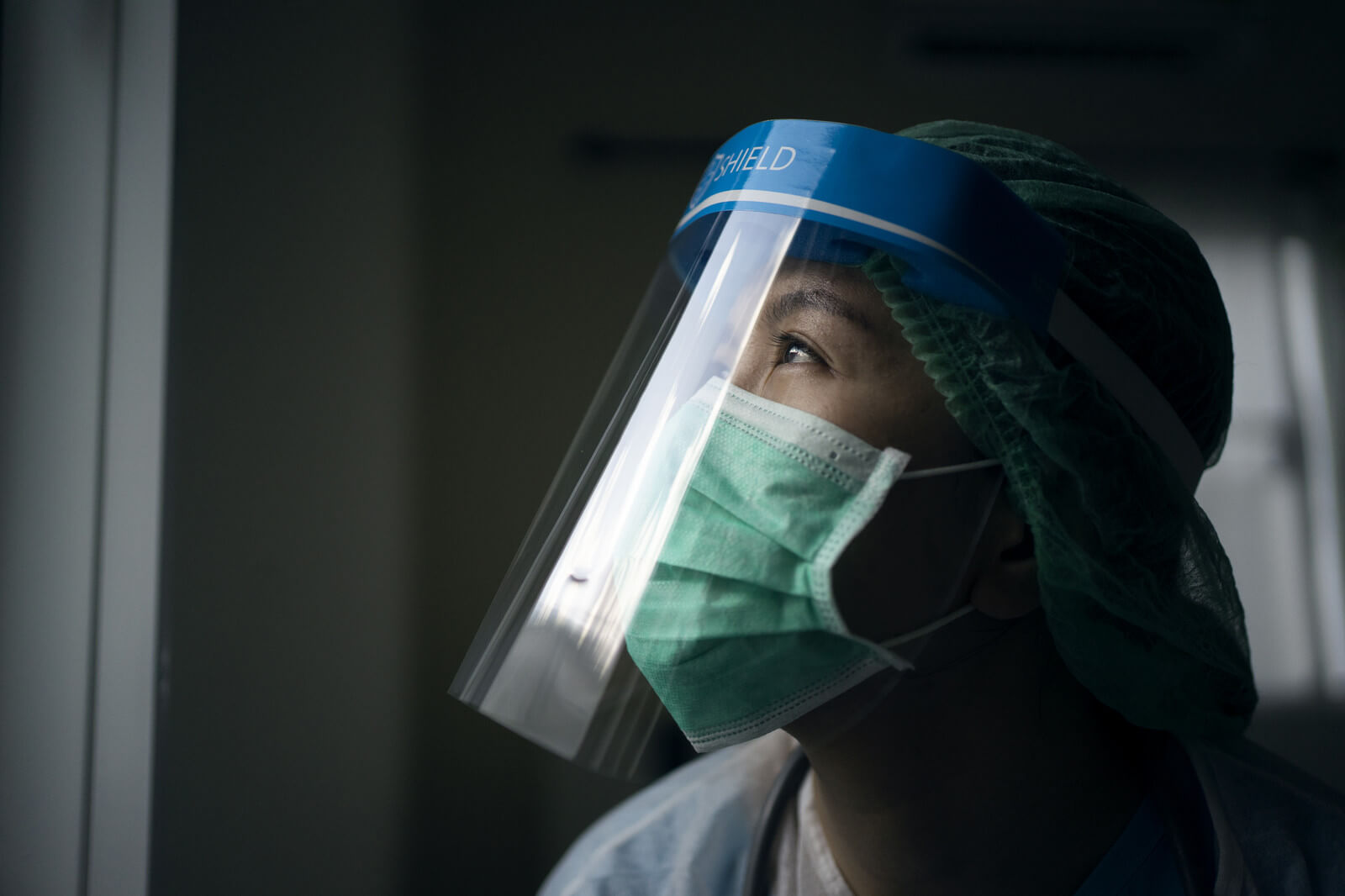
One year. That’s how long it has been since the World Health Organization declared COVID-19 a global pandemic. Sometimes it feels like the year went by in the blink of an eye. More often, however, it feels more like five years have gone by since many of us last gathered with loved ones, went to a movie theater or grabbed coffee with colleagues at the office. But regardless of how long it has felt to each of us, I think we can all agree it has been a year defined by loss, learning and an approaching liberation.
The loss has been staggering. As of today, more than 117 million confirmed cases of COVID-19 have resulted in approximately 2.6 million deaths around the world. Unfathomable and heartbreaking. And the losses go far beyond the death toll. The numbers of lost jobs, shuttered family businesses, missed school days and skipped medical treatments are emotionally, physically and financially devastating. It has made for an unprecedented global nightmare – one from which at times it felt we would never wake up.
But along the way, incredible learning was taking place, particularly in the effort to bring forward vaccines and therapies to address this tragic pandemic. We found new ways to use digital technology, to partner with regulators and to perform tasks in parallel rather than sequentially – all of which allowed our scientists and clinicians to advance the clinical trials for our COVID-19 vaccine with unprecedented speed, without compromising safety or quality. We watched as human ingenuity solved problems that we hadn’t even pondered just 12 months ago. At Pfizer, this included inventing new thermal shippers to keep our vaccine at ultra-cold temperatures while in transit, and rooms filled with innovative new equipment that didn’t even exist on March 11, 2020. I first saw these machines with my own eyes when we hosted President Biden at our facility in Kalamazoo, Michigan, three weeks ago, and it was a moment of great pride, wonder and joy.
Across the industry we learned just how powerful collaboration can be. Collaborations between Pfizer and BioNTech, and between Moderna and the National Institutes of Health helped deliver the world’s first two mRNA vaccines. Not only the first two mRNA vaccines to fight COVID-19, but the first two mRNA vaccines, period. And now the manufacturing collaboration between Johnson & Johnson and Merck will further accelerate the number of vaccine doses delivered to the world.
Because of all this learning, we are emerging from year one of the pandemic with a sense that liberation is on the horizon. We are seeing encouraging real-world data about our vaccine coming out of Israel and other countries. Through outstanding private-public partnerships, the vaccine is beginning to reach even the poorest of countries, with the first mRNA COVID-19 vaccine doses arriving in Rwanda last week. And as vaccinations accelerate around the world, you can see, hear and feel hope returning. It’s the type of renewed optimism one often experiences in the spring, but this time it’s about more than leaving behind a cold winter; it’s about surviving one of the scariest periods in the history of our world.
But we are not out of the woods. That’s why Pfizer supports the recent statements from health authorities around the world about remaining vigilant in the coming months. We need to continue to wear our masks, maintain physical distancing and frequently wash our hands until enough people are vaccinated to result in herd immunity. And we’re not letting up with regard to our science. We are beginning booster trials to address the continually evolving variant strains of the virus; we are running studies in additional populations such as pregnant women and children; and we are advancing antiviral therapies in the clinic to potentially help patients battling COVID-19.
In just 12 months, we have made the seemingly impossible, possible. And now we have an equally important, challenging but achievable task. When we come through the other side of this pandemic, we need to carry forward the learnings of the past year to make sure we are better prepared for the next one. That’s how we will honor the loved ones we have lost – and how we will ensure science will win.
![]()
Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by FDA, but has been authorized for emergency use by FDA, under an EUA to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals aged 6 months through 11 years of age. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b) (1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner.
02.02.2026
01.27.2026
01.13.2026
01.08.2026
01.06.2026