What Makes an RNA Vaccine Different From a Conventional Vaccine?
Vaccines are one of the greatest health interventions ever developed. They’ve been cited as being as important to keeping communities healthy as having access to clean water and safe sanitation.1
Through scientific investment and ingenuity, today we have multiple vaccine technology platforms that have helped us control and, in some cases, eradicate many healthcare challenges such as polio, river blindness, smallpox, and COVID-19, just to name a few.
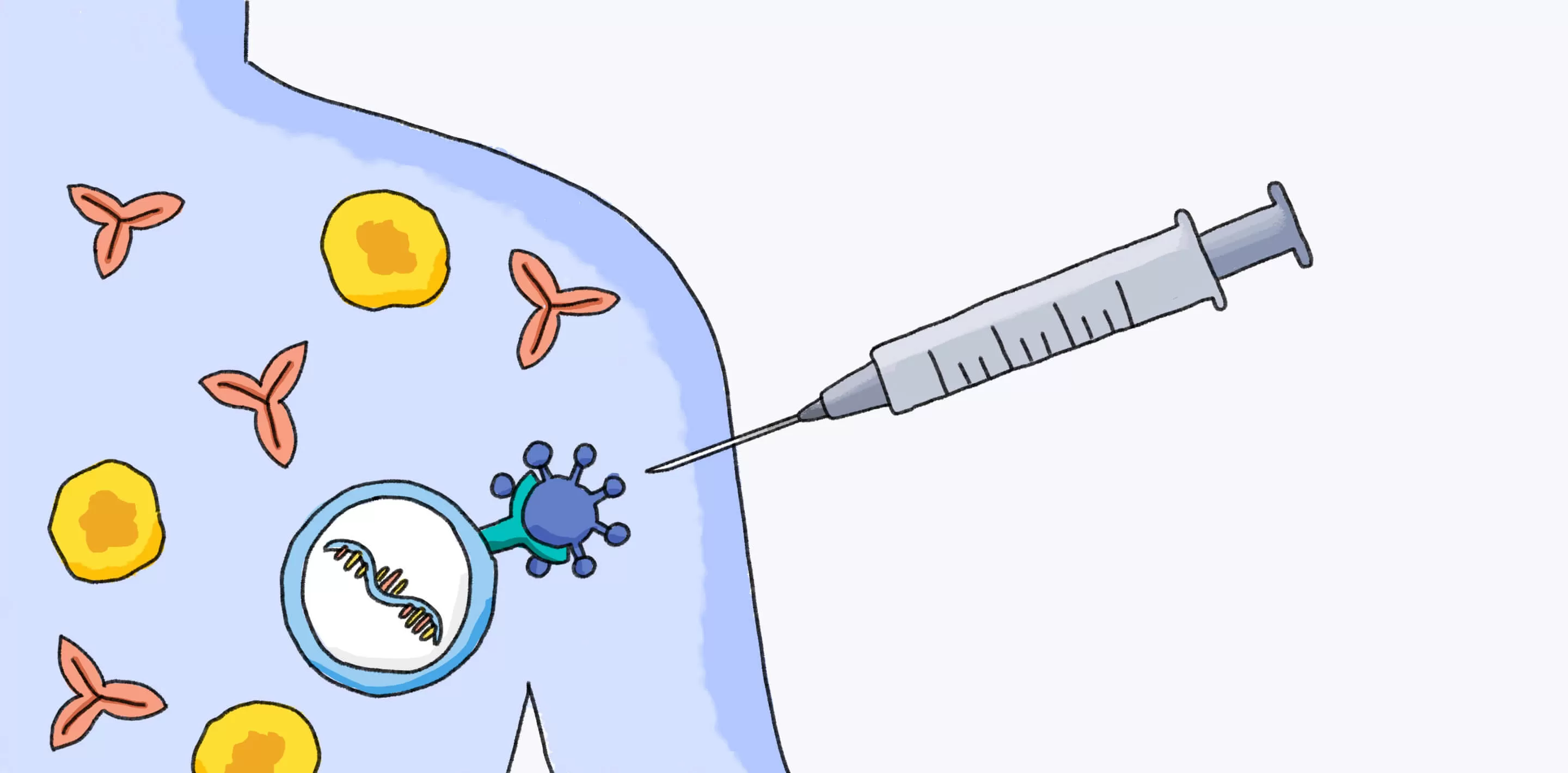
In 2020, messenger RNA, or mRNA for short, was the platform used to develop some COVID-19 vaccines, including the Pfizer-BioNTech COVID-19 vaccine. Unlike conventional vaccines, which can take months to produce by growing weakened forms of the virus, RNA vaccines can be constructed quickly using only the pathogen’s genetic code.
It is exactly those benefits that suggest to us that mRNA vaccine technology has the potential to provide more effective, more tolerable, and more convenient vaccines for diseases which don’t yet have mRNA alternatives, such as influenza and shingles.
Let’s take a closer look at the differences between traditional and mRNA vaccines.