Pfizer’s presence at ASCO 2024
Positive results from ECHELON-3 Phase 3 study
Regimen produced clinically meaningful improvement in overall survival for certain patients with relapsed/refractory diffuse large b-cell lymphoma (DLBCL).
Find Out MoreNew 4-year data in advanced classical Hodgkin lymphoma
Takeda and Pfizer announce positive four-year results from Phase 3 HD21 trial of combination treatment regimen in frontline classical Hodgkin lymphoma.
Find Out MoreNew data from CROWN study
Five year follow-up data shows prolonged progression-free survival in ALK-positive advanced lung cancer.
Find Out MoreUnderstanding ALK-Positive Advanced Non-Small Cell Lung Cancer
Background information about a rare, aggressive form of lung cancer called ALK-positive non-small cell lung cancer.
Find Out MoreBreaking new ground with lung cancer research
Advances in scientific research have brought new hope for patients with a rare, aggressive form of lung cancer.
Find Out MoreHighlighting progress in accelerating breakthrough cancer medicines at ASCO
Pfizer is showcasing new research across the company’s key tumor areas and core scientific modalities, including small molecules, antibody-drug conjugates and bispecific antibodies.
Find Out MorePfizer-sponsored abstracts at ASCO
More than 50 abstracts will be presented from Pfizer’s broadened portfolio of approved and pipeline therapies.
See Our Abstract ListPfizer Abstract Plain Language Summaries (APLS)
To help non-scientists better understand the latest research, we are providing abstract plain language summaries for key data at ASCO.
Find Out MoreDriving cutting-edge science forward
Pfizer leaders share progress toward our vision: a world where people with cancer live better and longer lives.
Find Out MorePress Releases
All Pfizer published press releases by date and/or category of news
06.12.2024 Research Pfizer Provides Update on Phase 3 Study of Investigational Gene Therapy for Ambulatory Boys with Duchenne Muscular Dystrophy 06.03.2024 Financial Finance Pfizer Invites Public to Listen to Webcast of Pfizer Discussion at Healthcare Conference 06.01.2024 Prescription Medicines Research Pfizer’s ADCETRIS® Regimen Produces Clinically Meaningful Improvement in Overall Survival in Patients with Relapsed/Refractory Diffuse Large B-cell Lymphoma (DLBCL)
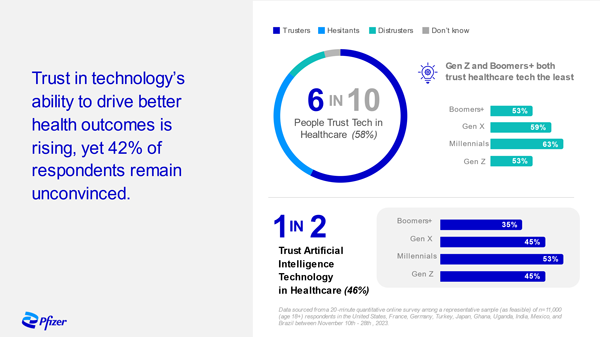
Trust in Technology – A Snapshot
Pfizer surveyed adults in 10 countries across the Global North and South to better understand the general population's trust in incorporating technology into the management of their healthcare. The results showed that 6 in 10 trust technology in this setting but that means 4 in 10 are at risk of being left behind. Our findings underscored that patients won’t feel the full benefits of that technology if we don’t have trusted people – specifically health care providers – delivering it. We must partner with patients, healthcare providers, policy makers and others in the life sciences and tech industries to build trust in the technologies that are fueling the future of health.
Downloadable InfographicDetails
Media Asset Library
A collection of assets for use by media.
Partnering News
The latest news from Pfizer and its strategic partners
11.28.2023 Corporate Medicines Arvinas and Pfizer Announce Updated Vepdegestrant (ARV-471) Data to be Presented at the 2023 San Antonio Breast Cancer Symposium 11.16.2023 Corporate Medicines Pfizer and Astellas' XTANDI® Approved by U.S. FDA in Earlier Prostate Cancer Treatment Setting
Media Contacts
Pfizer Media Relations
We encourage everyone to view our press releases, press statements, and press kits to stay up to date on Pfizer news.
However, to ensure that customers, investors, and others receive the appropriate attention, Pfizer media contacts may only respond to calls and emails from professional journalists.
Media Contacts
General email
[email protected]
United States, Canada and Latin America
+1 212.733.1226
Europe, the Middle East and Africa
+44-(0)173.733.2335
[email protected]
Asia Pacific
+65.918.732.47
What we need
To best help us help you, please be prepared to provide your name and email address, a summary of the article you're working on and your deadline.
Other Frequently Visited Links
For more, see these helpful and often used sections of Pfizer.com
Pfizer Wire
Stay up to date on the latest news and alerts through the Pfizer Wire
Sign up to receive real-time updates on Pfizer’s news from the Pfizer Media Relations team delivered directly to your inbox.
Sign Up NowDetails