Partnering with Patients
Do you know how medicines are studied?
Healthcare providers, pharmaceutical companies, regulatory authorities (like the FDA) and patients work together to better understand and reduce risks of medicines. This extensive safety system is called Pharmacovigilance (PV). Pfizer has thousands of experts around the globe working to analyze and communicate the risks and benefits of our medicines both before and after they are approved for use. Patients also have an important role to play in reporting side effects they may experience.
Patient Safety
Helping patients use their medicine more safely
Understanding the roles of patients, healthcare providers, pharmaceutical companies, and regulatory authorities1
You or a loved one will likely take medicine at some point in life.
82% of U.S. adults take at least one medicine,
including vitamins and supplements, in a given week2
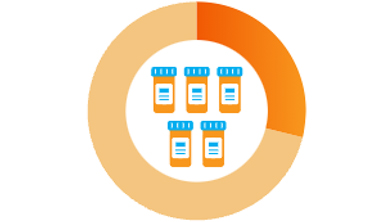
29% take five or more3
As a patient, you want your medical treatment to:4,5
 Improve your health Improve your health |  Be as safe as possible Be as safe as possible |
Safety is everyone’s first priority, but medicines may still have side effects.
Fortunately, there are many steps in place to help protect those who take medicines.
Balancing Benefit and Risk 4,6
Regulatory agencies review data and approve a medicine when its benefits are greater than its risks.
It takes studying thousands of possible new medicines by companies like Pfizer to get ONE through the drug development process and approved by regulatory authorities such as the FDA.7
Pfizer’s medicines are available in over 150 countries around the world. We employ thousands of medicine safety specialists. These specialists work with regulatory authorities to understand and communicate the risks and benefits of our medicines both before and after they are approved for patients.
Companies like Pfizer and regulatory authorities like the Food and Drug Administration (FDA) make every effort to understand:

Pharmacovigilance
What is pharmacovigilance (PV)?9,10
Pharmacovigilance is defined by the World Health Organization (WHO) as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem”
PV includes an extensive safety system
Identifying and managing possible safety concerns associated with the medicines we take


Helping protect patients
Drug companies, healthcare providers and regulators are always monitoring for medicine safety, both before and after a medicine has been approved.

Clinical trials often find common side effects of a medicine, but some effects can only be found when large numbers of people are using the medicine after it is approved.
What can you do
You, the patient or caregiver, are an important part of this network.10
Understand how to use your medicine more safely and its possible effects1

Read the product information and any other trusted resources available to you

Speak to your healthcare provider about your specific situation
Wherever you live, you should tell your healthcare provider if you experience any side effect. You can also tell the drug company. If you are in the U.S., you can contact the FDA (MedWatch).11,12

Realize you or loved one might be the first to experience a side effect. Please be sure to report any sides effects:
Report side effects for Pfizer products by calling 1-800-438-1985.


Contact the U.S. FDA directly at FDA.gov/Safety/Medwatchor call 1-800-FDA-1088.
To report side effects outside the U.S., ask your healthcare provider or local health authority for more information.

A key piece of the pharmacovigilance process is your own careful attention to side effects.10
SOURCES
1www.nia.nih.gov/health/making-decisions-your-doctor
2www.fda.gov/Drugs/ResourcesForYou/ucm079492.htm
3www.fda.gov/ForConsumers/ConsumerUpdates/ucm164616.htm
4www.your-heart-health.com/en-US/risk---prevention/assess-your-risk.html
5www.nsc.org/learn/safety-knowledge/Pages/injury-facts-chart.aspx
6www.ema.europa.eu/docs/en_GB/document_library/Report/2014/12/WC500178511.pdf
7www.fda.gov/RegulatoryInformation/Guidances/ucm127509.htm
8http://ecp.acponline.org/janfeb00/primer.htm
9https://newsinhealth.nih.gov/2016/10/understanding-health-risks
10www.fda.gov/drugs/resourcesforyou/consumers/ucm143566.htm
11Shepherd et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: A cross-over trial. Pat Ed Couns 2011; 84: 379-385.