The Great Migration: Tracking Immune Cells’ Travels
Scientists are studying how immune cells move to develop better treatments for autoimmune diseases.
Birds migrate. People migrate. And so do cells.
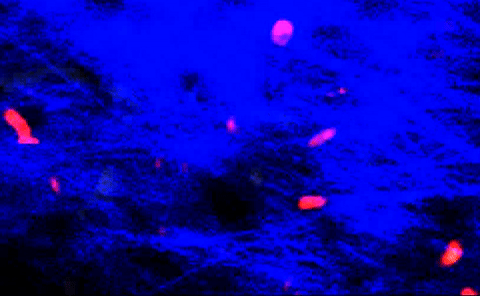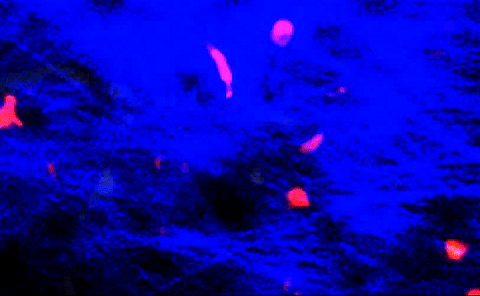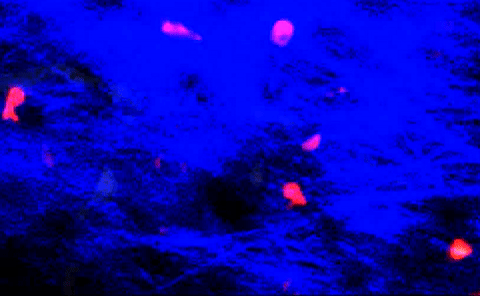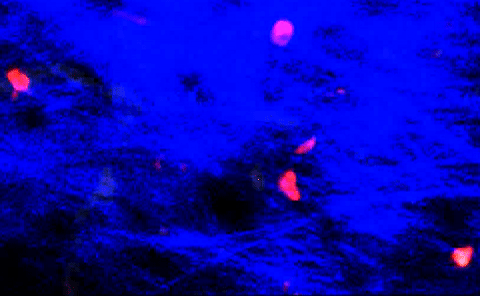
T-cells squeezing between collagen fibers. PLOS ONE/Coles JA
Our immune cells, in particular, are always on the move, constantly patrolling the body for foreign invaders. If an unwelcome visitor enters through the nose, mouth or eyes, or there’s a break in the skin, these nimble soldiers are ready to flood the attack site and send out signals for backup from other specialized immune cells. If the body’s first line of defense, known as the innate immune response, is unsuccessful in defeating an invader, the adaptive immune response sets in — usually within a few days — targeting the pathogens more specifically. The immune system is amazingly complex and made up of many types of cells, each with specialized functions, and various proteins that act as signals and activators during the immune response.
For reasons that scientists don’t fully understand, the immune system sometimes becomes confused, sending a fleet of fighters to attack the body’s own tissues and organs. The result is a range of autoimmune conditions from rheumatoid arthritis and psoriasis to Crohn’s disease and multiple sclerosis.
Itinerant Immune Cells
In a growing field known as immune cell migration, scientists like Pfizer’s Wei Li are closely studying the movement patterns of immune cells in autoimmune diseases and the cascade of events that lead these cells to attack one’s own body. More specifically, they’re examining the proteins that act as homing signals that attract immune cells to an inflammation site. “We’re looking for ways to block these proteins so the cells can’t migrate and cause inflammation and damage,” says Li, a Senior Principal Scientist in Pfizer’s Inflammation and Immunology research unit in Kendall Square, in Cambridge, Massachusetts.
The challenge in this field is the amazing complexity of the immune response and the varied steps that comprise an immune reaction. For decades, researchers have been studying different players in cell migration for potential autoimmune therapies. “The current understanding is that migration is a step-by-step process, and we’re looking to find the disease-causing step and target it. Looking back, the initial years of cell migration therapy research and development were largely trial and error, but the knowledge generated during that time is guiding us to better targets and better approaches today,” says Li.
Selective Suppression
Li and colleagues are looking at ways to block T-cells — the immune system’s main soldiers — from flooding the skin and joints and forming pockets of cells that become inflammation sites in conditions such as psoriasis and inflammatory bowel diseases. The goal is to block a specific protein that signals the migration of T-cells and the migration of dendritic cells (an immune cell that triggers the activation of T-cells) to the site.
“We’re trying to suppress the T-cells from getting to the joint or tissue, clustering to form a disease center,” says Li. “If they don’t aggregate with other immune cells, there is much less of a chance for them to proliferate."
They’re also looking for specificity: The hope is to produce a small-molecule medicine that targets a specific subtype of T-cells causing these autoimmune conditions while sparing the rest of the immune system. “Some existing therapies stop every type of T-cell and have side effects. We’re finding different ways to tackle a problem to give patients a better option,” says Li.