Better, Faster, Smaller: How an Advance in Microfluidics Can Speed Up Drug Discovery
Before preparing a new recipe for 20 guests at Thanksgiving, the smart cook starts small, making the dish for their immediate family to test things out before the big holiday dinner. What does a scientist do when attempting to discover a molecule that has the properties that potentially could be a miracle cure for diabetes, high blood pressure or another troublesome disease? She also starts small, testing the molecule to make sure it’s safe and effective in living cells.
Increasingly, this means using tiny devices measured in mere centimeters as an early part of the testing process. These devices contain carved miniature chambers to hold cells or tissue samples from different parts of the body. In addition, some of these devices are even designed to mimic aspects of the architecture, nutrient environment, and mechanical forces experienced by these cells or tissues in their native physiological environment. If a scientist wants to test the potential toxicity of a substance on, say, the liver, a small sample of liver cells can be incorporated into the device. The experimental molecule then flows through the device and encounters the liver cells in a manner that recapitulates what would happen in an actual living organism.
A key to translating this type of in vitro testing to actual patients is the science of microfluidics—the manipulation of fluids that are constrained to a scale where the longest distances are measured in millimeters.

Derek Bartlett
“Microfluidic devices have the potential to improve our ability to create potential medicines for human patients with a reduced reliance on animal testing,” says Derek Bartlett, PhD, a principal scientist in Pfizer’s Medicinal Sciences group who specializes in pharmacokinetics, dynamics and metabolism. “But these devices also have some limitations that must first be overcome before this can be made a reality.”
So, how can scientists address these limitations? One way is to invent a new microfluidics-based platform that allows researchers to incorporate dynamic drug exposure profiles when they work with microfluidic devices. Scientists at Pfizer and the microfluidics biotechnology company Neofluidics are working together to develop this kind of device.
Just as a flight simulator can help pilots hone their skills before taking to the sky in an actual airplane, the hope is that microfluidic devices may allow drugs to be refined and optimized before being given to animals for testing or eventually human patients for treatment. For example, when someone takes a medicine for cancer, the goal is to kill off the cancer cells, while sparing as many of the healthy cells as possible. But how much drug is required and how often must it be taken to strike this delicate balance?
A key challenge in the drug development process is understanding the interplay between a drug’s exposure, efficacy, and toxicity. This is a complicated undertaking, because when a person’s body processes a molecule, the body makes important changes to the molecule that may affect how it works. The study of how an organism affects a medicine is called pharmacokinetics (PK). The study of how the drug affects the organism is called pharmacodynamics (PD).
Together, PK/PD models can provide information on the effects of different doses of a molecule, how quickly a substance is broken down by the body and other important elements that influence the safety and efficacy of a medicine. We call this the ‘exposure profile’ of a molecule.
“We really need to evaluate nuances when we look at the exposure profile of experimental molecule so we better understand the safety and efficacy of the molecule when we move on to human studies,” says Bartlett.
The Pfizer-Neofluidics partnership is anticipated to create a first-of-its-kind microfluidics-based platform to study dynamic PK and PD profiles in vitro (meaning in the laboratory before treatment in living organisms — either animals or human patients).
“We want to be able to answer questions like, ‘How long do we need a molecule to stay above a certain exposure level to be effective?’ or ‘How can we tailor a molecule’s properties to stay on target for more or less time to achieve this desired exposure profile?’” says Bartlett, referring to a medicine’s ability to either continue or stop making the intended change to a person’s body. “We hope that microfluidic devices that simulate a human PK profile and monitor the PD response will help us answer these kinds of questions.”
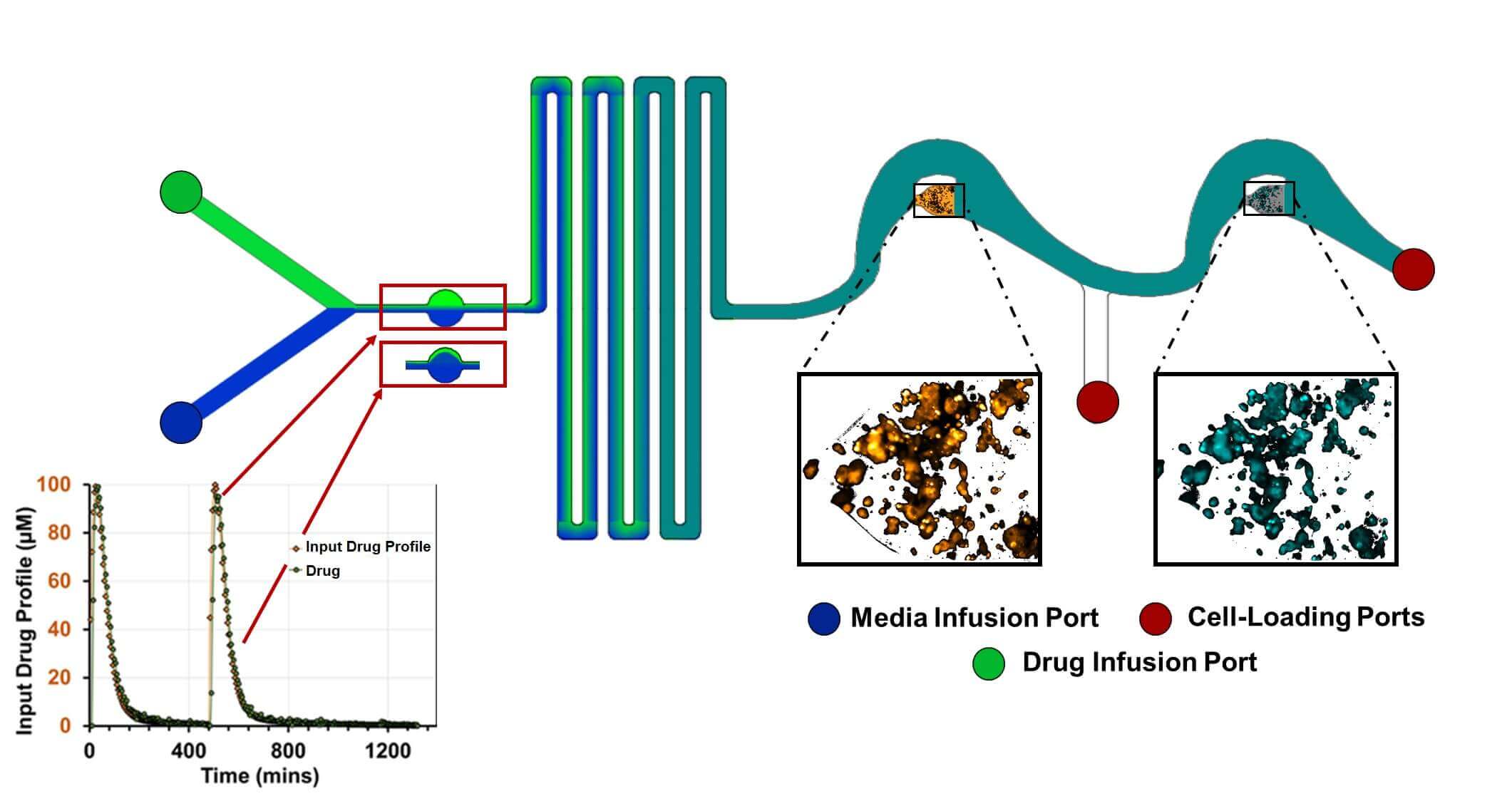
Schematic of a microfluidic-based PK/PD (NeoPKPD) device. A computer-controlled pump establishes the proportion of drug-containing fluid added to the system over time via the indicated infusion ports, enabling the downstream wells to be exposed to the drug in a manner that mimics the drug’s physiologically relevant pharmacokinetic (PK) profile that is observed in blood. The wells in the device can be designed to use samples ranging from single layers of cells to 3D spheroids or tissues. The pharmacodynamic (PD) response can then be monitored using microscopy, direct sampling, or extraction of the well contents. Figure courtesy of Neofluidics.
We also anticipate that these devices potentially may also help speed up the process of drug discovery and testing in a couple ways. First, by modeling a human drug PK profile on a laboratory device before modeling it on a real person means that scientists can do in-depth studies on a potential new medicine in a setting where they have unprecedented control. This added control, if successful, is expected to reduce uncertainty and improve efficiency of the experiments. Second, it is hoped that an in vitro microfluidic platform will mimic human PK profiles and be able to monitor reactions to a selected dosing regimen before testing on humans begins, potentially improving the safety for research subjects in clinical trials and saving time in reaching a recommended dose for efficacy.
Ultimately, a microfluidics-based platform that allows for the study of dynamic PK and PD profiles is a potential game-changer in drug development. Microfluidic-based devices have already made a major difference in how scientists test molecules in the lab by allowing researchers to test how new molecules will affect cells. If they can also be tweaked to account for the dynamic drug exposures that those same cells experience, these little devices could be the next big thing in drug discovery.