Growing a Tiny Gut

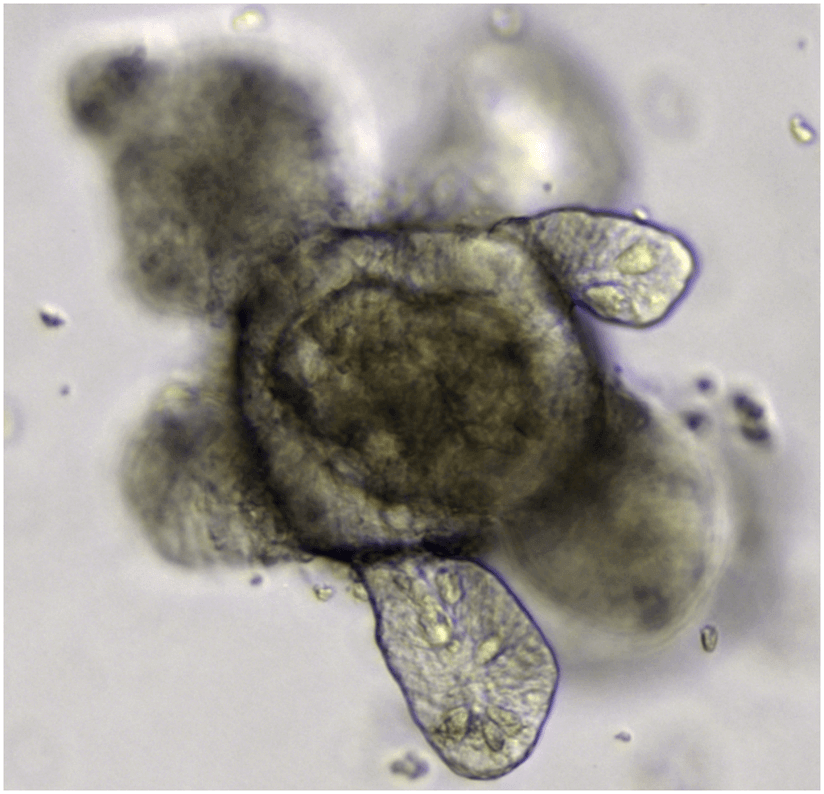
Creating a miniature intestine in a lab — called an organoid — can help researchers see how real guts will react to disease and medicine.
Organoids sound like the stuff of Dr. Frankenstein’s mind — body parts grown in labs, created for scientific experimentation. What they are in reality are living tissues, grown from seed cells, that can tell researchers a lot about the way human organs will react to diseases and the medicines that may treat them.
Tiny Titans
Though some researchers are growing full-size organoids (like the brains at the Institute of Molecular Biotechnology in Vienna, Austria), Celia Caballero-Franco, part of Pfizer’s research team at Kendall Square, Cambridge, is growing intestinal specimens that are tiny, just a mere millimeter in size.
But that’s big enough to carry the characteristics and abilities of a real gut. It’s not a mimic. It’s the real thing, wrought in miniature. There are villi to push food through, cells that produce mucous for lubrication, and cells that secrete chemicals that stimulate the contractions that keep things moving. It can even transport nutrients, just like a real gut.
By way of comparison, an adult human’s intestines, which live fold upon fold in the lower part of the abdomen, are about 25 feet long in total, 20 feet of small intestine and five feet of large intestine (so-called because of its girth, not length). That’s about the same length as half a badminton court. “All that is just to absorb nutrients,” says Caballero-Franco.
Making Information Digestible
Until organoids, most lab experiments on living cells were done in 2-D. A cell sat on top of a culture in a petri dish and proliferated. That wasn’t a great representation of the way a real organ would react, though. An intestine is more than a collection of cells; it’s an organized system. So the behavior of a pile of cells wouldn’t necessarily reflect how a full-fledged organ would react.
But organoids are real working organs, created from some stem cells of the real thing. In the case of the guts in Caballero-Franco’s lab, that means mouse cells. But human cells could be harmlessly harvested in the future.
Such human organoids could potentially help personalize treatment, for gut ailments from irritable bowel syndrome to colon cancer. Cells from a patient with cancer, for example, could be treated outside a person’s body with all the various options available, saving the patients from the side effects of treatments that aren’t effective and honing in on the medicines that will help them most before they’ve ever taken a dose.
Multiple and miniature samples mean that many treatments can be tested simultaneously and in a minimum of space. And gut organoids are self-propagating — one poke to the central body causes each of the fingerlike projections (see image above) to break off, forming their own baby gut, which can happen about every five days. This process can happen ad infinitum, so in essence organoids can be cultured indefinitely, making one harvest of seed cells all it takes to create an abundant supply of testable guts, letting researchers replace any that die off.
The organoids are being grown currently to study how organs work, not replace them. Though in the future, the tiny guts could potentially also be used as patches, say, to fix a hole made by an ulcer.
—Johnna Rizzo