International Project to Help Detect a Liver Disease Called NASH Gets $35M Boost
Imagine if the only way to know whether you have diabetes was for a doctor to take a biopsy of your pancreas, rather than being able to check your glucose levels using a simple blood test.
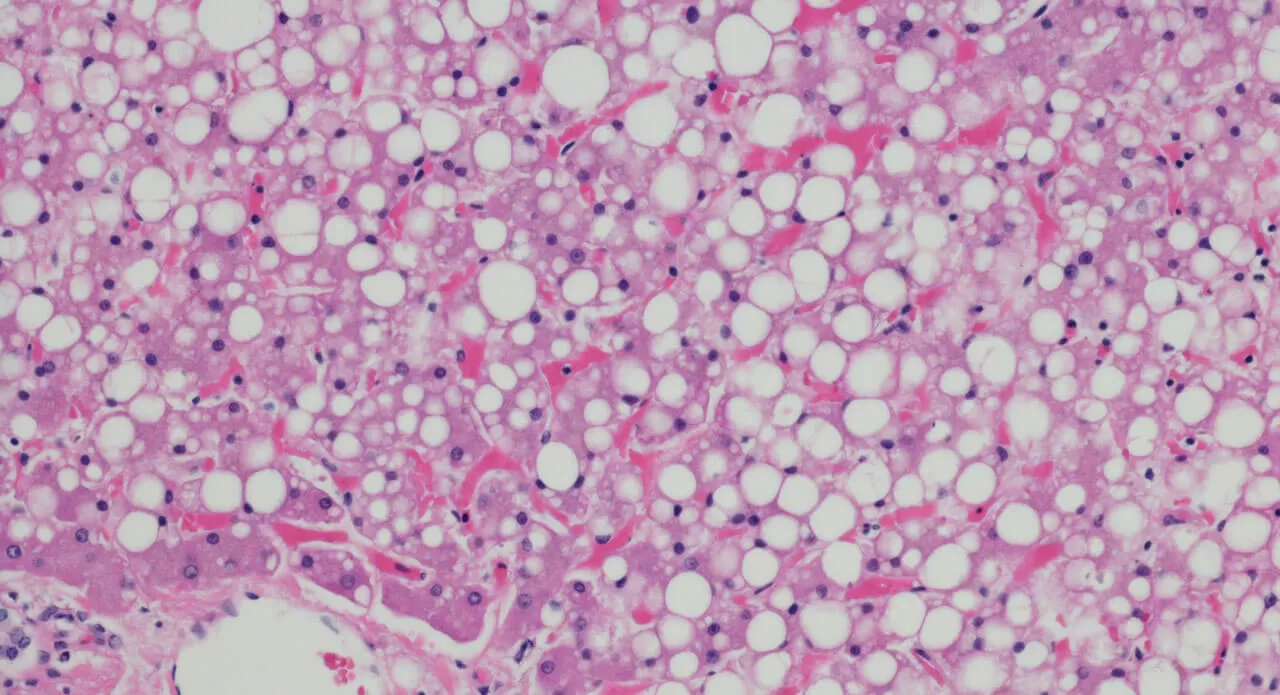
That is the current state of affairs for a disease known as NASH (non-alcoholic steatohepatitis) in which the liver is damaged by inflammation due to a buildup of fats. NASH is a progressive subtype of non-alcoholic fatty liver disease (NAFLD). Associated risk factors for NAFLD, NASH and the later stages of NASH (liver fibrosis and cirrhosis) are obesity, Type 2 diabetes, and the collection of symptoms known as metabolic syndrome.

Dr. Julia Brosnan, Senior Director of External Collaborations and Scientific Alliances
But an ambitious new project, involving a consortium that is part of the Innovative Medicines Initiative (IMI), the world’s largest public-private partnership of its kind, aims to tackle the glaring lack of diagnostic tools by seeking reliable, measurable medical signs, or biomarkers, that indicate the presence of NASH. The project, co-led by Pfizer, is known as LITMUS, or “Liver Investigation: Testing Marker Utility in Steatohepatitis.”
“LITMUS is a game-changer,” says Dr. Julia Brosnan, Senior Director of External Collaborations and Scientific Alliances for Pfizer, one of 47 academic, clinical and pharmaceutical industry partners in the consortium, which is part of the IMI public private partnership. “None of us can do this on our own.”
LITMUS is coordinated by Professor Quentin Anstee at Newcastle University in the U.K. and is part of IMI, which is funded jointly by the European Commission and the European pharmaceutical industry, known by its acronym, EFPIA. LITMUS has an overall budget of €31.6 million (about $35.6 million) with pharmaceutical partners and the European Commission each providing €15.8 million euros for the five-year project.
NAFLD affects about 25 percent of the population around the world, according to a 2016 study. “[A] challenge for doctors is identifying which NAFLD patients will go on to develop NASH and, for those with NASH, predicting how fast the disease will progress,” as was stated in IMI’s call for proposals for the project.
However, the symptoms of NASH are often hidden for a long time. “Meanwhile the damage is accumulating inside the body,” Brosnan notes. “You really don’t know until it’s very late down the line. And that’s why the non-invasive diagnostics are so important.”
“Currently, you can only diagnose an individual with NASH if you can take a biopsy. And there needs to be a clinical need to take a biopsy,” Brosnan says.
The main way that damage manifests itself in the liver is something called fibrosis— a thickening and scarring of the tissue that can ultimately lead to cirrhosis and liver failure.
“There’s emerging evidence now that levels of fibrosis in the liver really have a big impact on your risk of dying from liver-related outcomes,” Brosnan says.
Another Reason Diagnostic Tools for NASH Are Needed
The other major issue with not having non-invasive diagnostic tools is that even as treatments for NASH are currently being developed, there is no way to check how effective those treatments would be without doing a biopsy— which can be a painful procedure that also incurs the risks associated with minor surgery.
“As we see therapies being developed, we want to have the tools out there to get to patients who might actually need it,” Brosnan says.
It’s believed that NASH is currently underdiagnosed, she adds, noting that one reason is because there’s no therapy yet, so even if a doctor were to diagnose someone with NASH, there would be no treatment, other than to tell the patient to eat healthier and exercise more. “We already tell the patients that anyway,” Brosnan says.
What’s Next in the NASH Biomarker Project
The seeds of the initiative were planted in April 2016 when the IMI put out a request to the academic and clinical communities to pool what turned out to be more than 1,500 biopsy samples scattered over multiple locations. The average hepatologist—clinicians who deal with the liver—has at most dozens of samples, Brosnan notes.
“It’s been a hodgepodge of smaller studies that have never really been harmonized, and by analyzing the data in a concerted fashion, we think there will be some clues as to which kinds of biomarkers will inform us,” Brosnan says. “The field is moving very rapidly right now. It’s getting to be exciting when you go to the conferences and realize that big steps are being taken.”
Most experts in the field expect that it won’t be one single biomarker that is used as a diagnostic tool, she says, but more likely a combination of a blood or other circulatory biomarker and an imaging biomarker.
An example of a blood biomarker is the blood glucose level used for diabetes. An example of an image biomarker is a chest X-ray for tuberculosis.
“We need biomarkers that tell us the state of the disease, but also biomarkers that tell us which of the treatments are working well,” Brosnan says of the need for the NASH biomarker initiative. “It’s a very exciting project.”