What are ADCs?
Targeting cancer
with special drug
delivery units
For centuries, humanity has hoped for a world where people with cancer live better, longer lives – and today that vision is closer than ever.
Scientists predict that an emerging powerful cancer therapy called antibody drug conjugates (ADCs) may replace the standard of care regimens for many cancers.1 While the first ADC was approved in 2000, several more are now available and rapid advancements in technology are providing the opportunity for ADCs to further improve outcomes for patients.2
ADCs are innovative cancer medicines that are designed to specifically target cancer cells and deliver cancer-killing drugs directly inside a cell while limiting damage to healthy cells. This technology may make cancer treatment more effective and help reduce side effects.
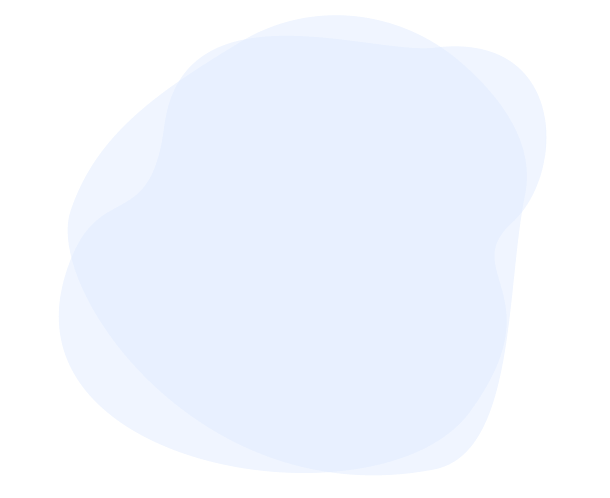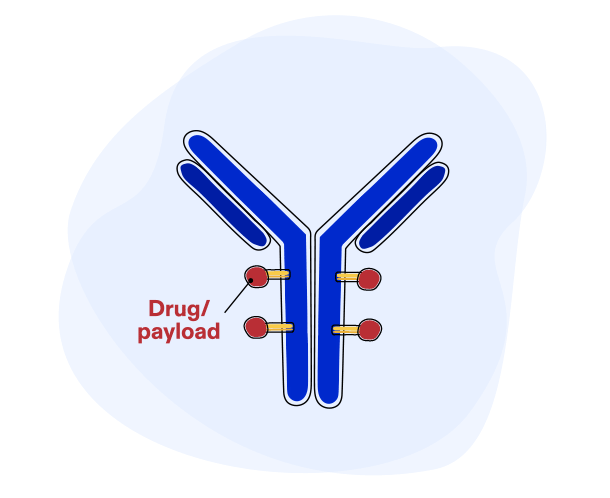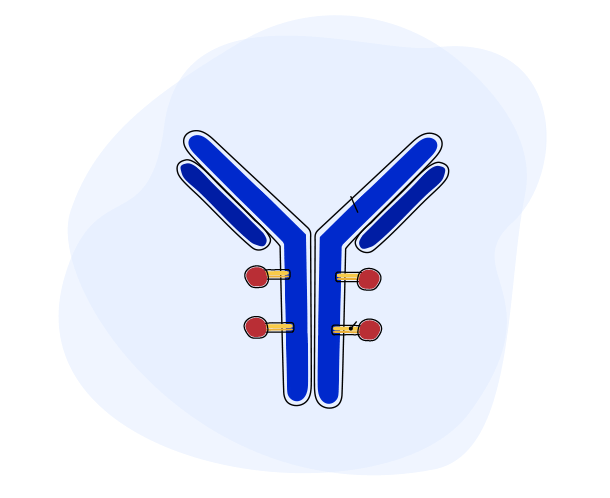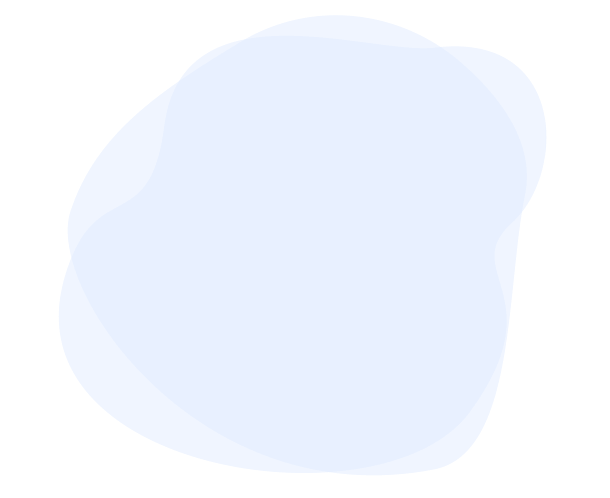
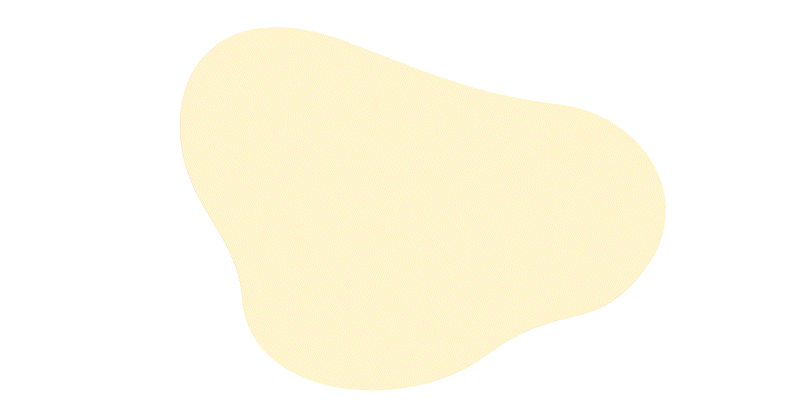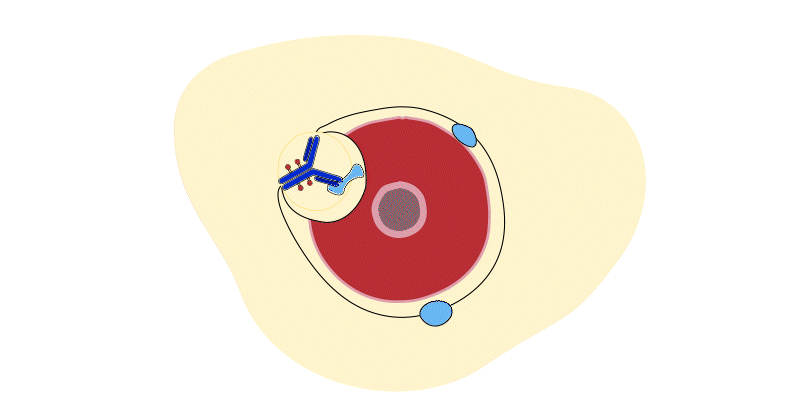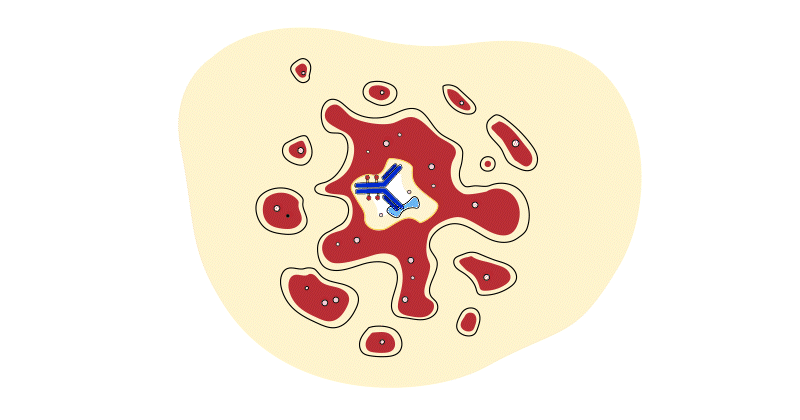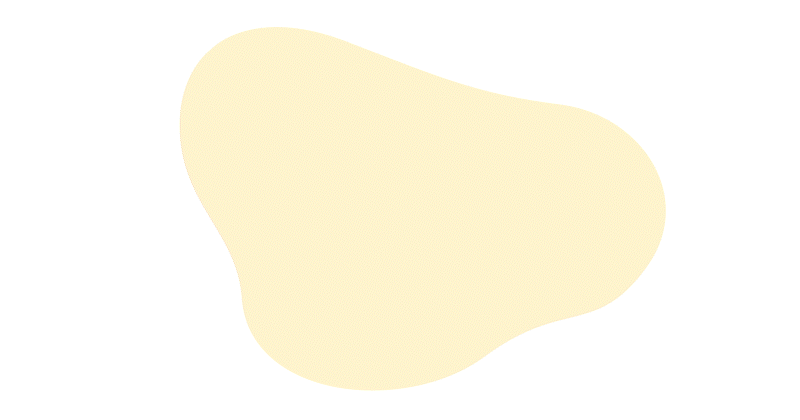
Each ADC has 3 parts:
- The payload, a cancer-killing drug that destroys the cancer cells once delivered inside a cell
- A monoclonal antibody, a specially-designed protein that guides the ADC to the right target – cancer cells – while minimizing impact to healthy cells
- A linker, which connects the payload to the antibody. Once inside the cell, the linker breaks apart, releasing the payload and minimizing damage to healthy cells
ADCs: A Unique Method of Action
Think of ADCs as special drug delivery units. They deliver cancer-killing drugs directly to tumors.
Unlike ADCs, traditional cancer treatments, such as chemotherapy and radiation, are not targeted, which means they can affect both cancerous and healthy cells. These treatments may also suppress the immune system.
ADCs release cancer-killing drugs inside cells with corresponding identifier, so patients can receive drugs more efficiently directed to cancer cells while limiting harm to healthy cells. Patients typically experience fewer treatment-related side effects, potentially meaning less disruption to their daily lives. And because ADCs do not suppress the immune system, ADCs can be combined with other treatments, like immunotherapies, to potentially improve their effectiveness and help overcome treatment resistance.
ADCs have improved survival for some types of cancer and can also be an option for people with certain cancers who might otherwise be out of treatment options
ADCs Are Approved to Treat:7,8,9

ADCs are emerging as one of the most effective tools in cancer treatment. One of the key challenges when treating cancer is that many therapies are not able to discriminate between cancer cells and healthy cells, potentially leading to side effects for people living with cancer. ADCs are designed to address that challenge by functioning like guided missiles that zero in on cancer cells to deliver powerful medicines specifically to those cells, while limiting damage to healthy cells.
The human body is normally well equipped to respond to external threats. The immune system springs into action when viruses or bacteria enter your body. It makes antibodies that are specially designed to attack and destroy invading viruses or bacteria. Sometimes, your immune system wipes out a threat before you even feel sick.
But cancer develops within our bodies. And by the time cancer is detected, it may have already evolved mechanisms that help it “hide” from the immune system.
Through research, scientists have learned that certain markers (or identifiers) are present on the surface of some cancer cells (and limited or absent on other healthy cell types). They’ve also learned how to create molecules that seek out and bind to those markers.
The antibody part of an ADC is engineered specifically to recognize and attach to these markers (called antigens) found on the surface of cancer cells. Think of a magnet: A magnet will attach tightly to a steel ball, but it won’t stick at all to a rubber ball.
Using these specific markers, ADCs can seek out and then enter cancer cells. Once inside a cell, enzymes break down the linker, releasing the ADC’s payload. The cell dies, leaving behind debris that may attract the attention of the immune system.
The Future
ADCs are emerging as one of the most powerful tools in cancer treatment. Scientists are currently working to refine this proven technology, with the potential to create a broader range of targeted treatments and more options for effective care.
At Pfizer, we are building off our strong history in ADCs to continue to develop treatments that can make a meaningful difference for patients. We are leveraging our deep ADC development expertise to target both known and new cancer cell markers, while also testing different types of cancer-killing drugs, creating the next frontier of ADCs.
Over the last decade, we’ve worked to increase potency and minimize off-target toxicity with ADCs, helping them move into frontline treatment in multiple cancer types. Now, we’re focused on expanding the potential of ADCs to make them even more effective and targeted.

Through our work today, we are building on our institutional knowledge to create the next wave of ADCs that are even more effective and targeted. With a robust pipeline and diverse research approaches, we believe we are uniquely positioned to bring forward the most innovative ADC portfolio in the industry to help people with cancer live longer and better lives.
References:
1Willams HR, Singh S, Birrer MJ. New Developments in Antibody-Drug Conjugates for the Treatment of Ovarian and Urothelial Carcinomas. ASCO Daily News. March 22, 2023. Accessed June 23, 2025. https://dailynews.ascopubs.org/do/new-developments-antibody-drug-conjugates-treatment-ovarian-and-urothelial-carcinomas.
2Metrangolo V, Engelholm LH. Antibody-Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers (Basel). 2024 Jan 20;16(2):447. doi: 10.3390/cancers16020447.
3Pettinato MC. Introduction to Antibody-Drug Conjugates. Antibodies (Basel). 2021 Oct 27;10(4):42. doi: 10.3390/antib10040042. PMID: 34842621; PMCID: PMC8628511.
4Marmé F. Antibody-Drug Conjugates for Breast Cancer. Oncol Res Treat. 2022;45(1-2):26-36. doi: 10.1159/000521499. Epub 2021 Dec 16. PMID: 34915488.
5Colombo R, Tarantino P, Rich JR, LoRusso PM, de Vries EGE. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024 Nov 1;14(11):2089-2108. doi: 10.1158/2159-8290.CD-24-0708. PMID: 39439290.
6Kondrashov A, Sapkota S, Sharma A, Riano I, Kurzrock R, Adashek JJ. Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload. Pharmaceutics. 2023 Aug 19;15(8):2160. doi: 10.3390/pharmaceutics15082160.
7Liu K, Li M, Li Y, et al. A review of the clinical efficacy of FDA-approved antibody‒drug conjugates in human cancers. Molecular cancer. 2024;23(1). doi:https://doi.org/10.1186/s12943-024-01963-7
8Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers (Basel). 2023 Jul 30;15(15):3886. doi: 10.3390/cancers15153886.
9Furlow M, Corridon C, Coyle B, and Bonnot R. Oncology’s next revolution: Antibody-drug conjugates and how to push them into the future. ZS. March 12, 2024. Accessed June 23, 2025. https://www.zs.com/insights/oncology-antibody-drug-conjugates-revolution